Discussing Therapies Currently Available in High-Grade Epithelial Ovarian Cancer
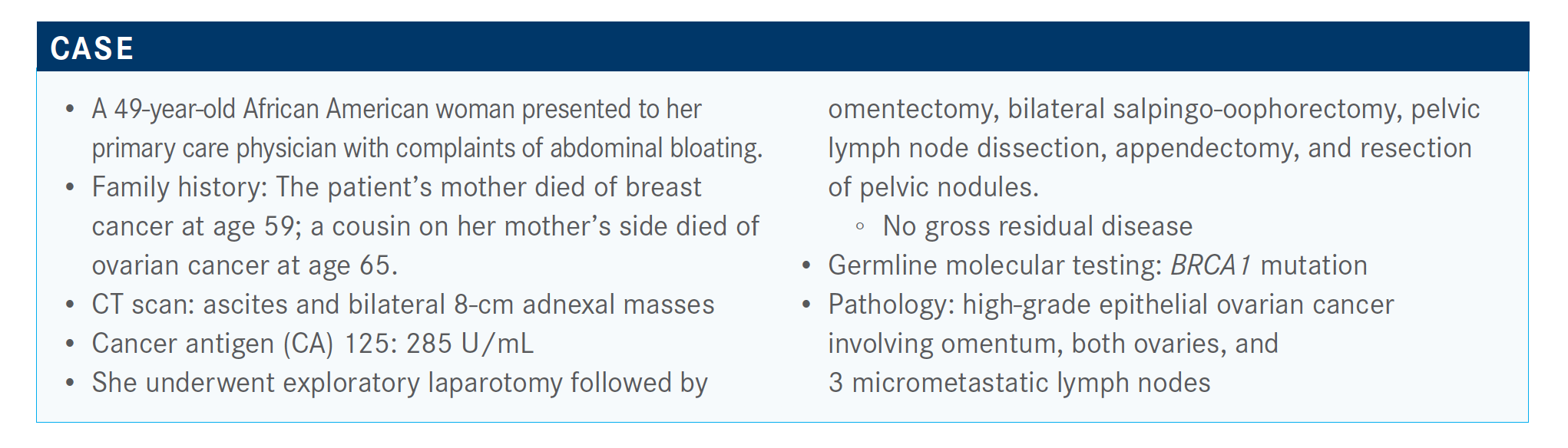
During a Targeted Oncology Case Based Peer Perspectives event, John K. Chan, MD, reviewed the case of a 49-year-old African American woman with high-grade epithelial ovarian cancer.
John K. Chan, MD

During a Targeted Oncology Case Based Peer Perspectives event, John K. Chan, MD, director, Gynecologic Cancer Research and associate clinical professor, Obstetrics/Gynecology, Reproductive Sciences, UCSF Helen Diller Family Comprehensive Cancer Center, reviewed the case of a 49-year-old African American woman with high-grade epithelial ovarian cancer.
Targeted Oncology™: Would you order additional molecular testing for this patient?
CHAN: If the patient is germline positive, I wouldn’t order another molecular test. I know how to treat this patient. I’ve got a piece of the tumor saved, and I can always reorder Foundation [Medicine testing] when the patient recurs.
What are the options for therapy in this patient?
If you use olaparib [Lynparza] alone, the median progression-free survival [PFS] benefit is 56 months.1 Maybe I won’t even need to worry about testing that tumor. She already has the ability to receive olaparib, so I generally don’t do additional molecular tests on these patients.
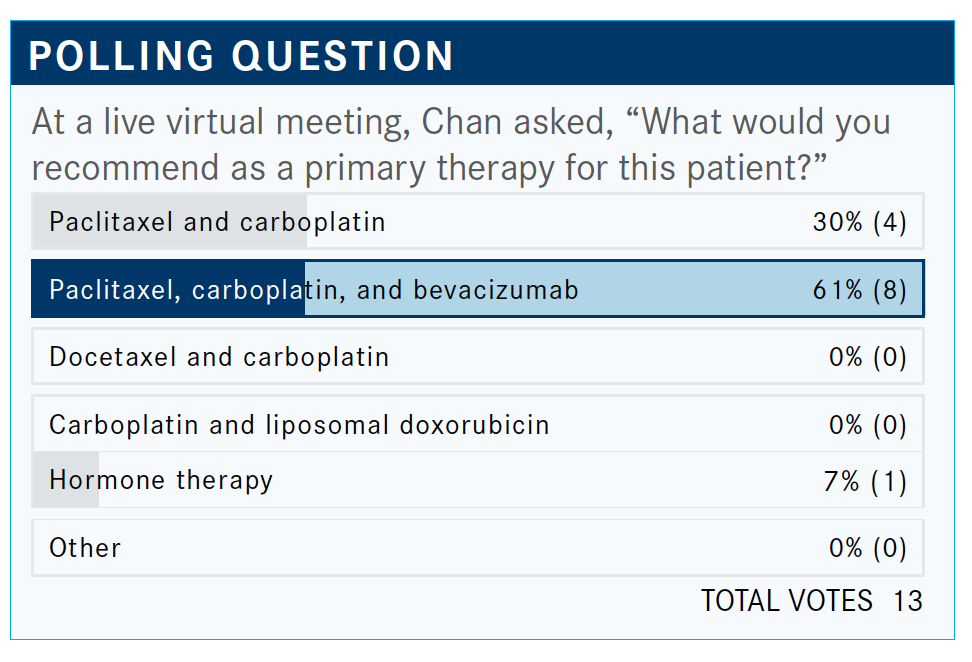
What do you think of the poll results?
Adding bevacizumab [Avastin] is a primary therapy for ovarian cancer. This needle is moving a lot as we speak, with all these combinations [incorporating bevacizumab] starting to come up. We are understanding more and more that ovarian cancer [therapy] is a maintenance strategy. This is very important.
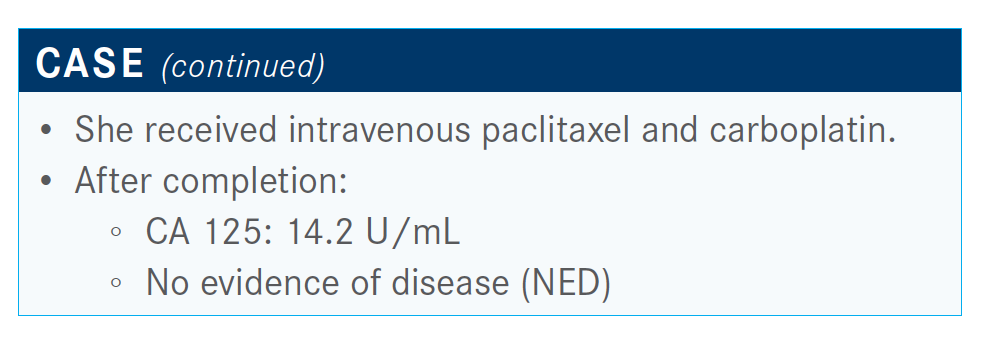
What would you recommend at this point for this patient?
[Most physicians] would go for olaparib because of the SOLO-1 trial [NCT01844986] and because she had germline-positive BRCA gene mutation.2 She was treated with chemotherapy and had a great response, resulting in NED. [This patient is similar to those] that ended up getting the PFS benefit of 50-plus months.
What factors influenced your decision to treat a patient such as this with a PARP inhibitor?
The toxicities. The magnitude of benefits associated with these PARP inhibitors have rocked our world and changed what we do.
References:
1. Banerjee S, Moore KN, Colombo G, et al. Maintenance Olaparib for patients (pts) with newly diagnosed, advanced ovarian cancer (OC) and a BRCA mutation (BRCAm): 5-year (y) follow-up (f/u) from SOLO1. Ann Oncol. 2020;31(suppl 4):S613. Abstract 811MO. doi:10.1016/j.annonc.2020.08.950
2. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505. doi:10.1056/NEJMoa1810858