Ovarian Cancer Benefits From Molecular Testing for Appropriate Therapy Designation
During a Targeted Oncology Case Based Peer Perspectives event, Bradley Monk, MD, discussed how the use of molecular testing can improve treatment decisions for ovarian cancer.
Bradley Monk, MD

During a Targeted Oncology Case Based Peer Perspectives event, Bradley Monk, MD, professor and director, Division of Gynecologic Oncology, Creighton University School of Medicine, St. Joseph’s Hospital and Medical Center, and medical director Gynecologic Oncology Research US Oncology Network discussed how the use of molecular testing can improve treatment decisions for ovarian cancer.
Targeted OncologyTM: How do you interpret these poll results? MONK: Somatic BRCA [testing] includes a homologous recombination deficiency [HRD] test…regardless of whether you choose Foundation Medicine or the Myriad test. Either tells you if [the tumor is] HRD because of the somatic BRCA or an epigenetic phenomenon.
Those of you who may not have said over 75%, you are probably the only honest people. I get it. This is molecular medicine, and it took a long time in the lung space [to get approval] for ALK and EGFR [inhibitors] and for checkpoint inhibitors for PD-L1 expression. It takes time for this to happen for KRAS mutations. But I think you guys being primarily a medical oncology–focused group, you get it.
Which laboratory test do you primarily use for tumor BRCA and HRD testing?
Myriad myChoice, and I like the FoundationOne CDx test as well. The reason people order Ambry Genetics Corporation’s TumorNext-HRD is because on 1 piece of paper you can do [more than] 1 test at the same time. People like Caris Molecular Intelligence because you get an entire molecular intelligence [report] including immunohistochemistry, which includes tests for microsatellite instability–high and all of that. People like Tempus for some of those same reasons. Memorial Sloan Kettering Cancer Center and Dana-Farber Cancer Institute are doing it in-house. But there is no right answer here.
What are the therapeutic options for patients with advanced ovarian cancer that is BRCA wild type and HRD? How do you choose between options?
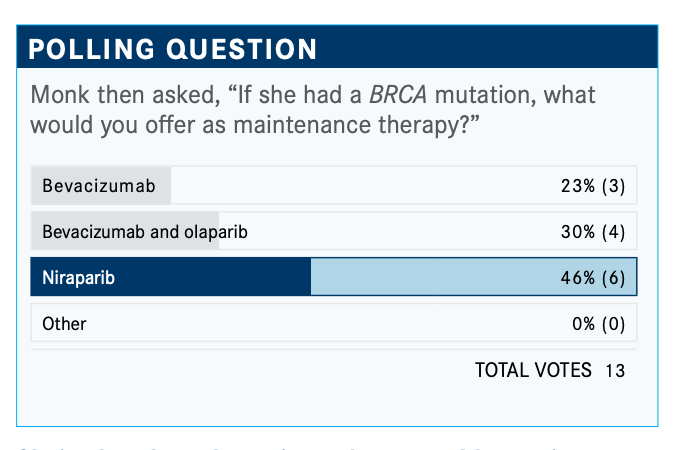
[One choice is] niraparib [Zejula], or if you’re doing bevacizumab [Avastin], bevacizumab and niraparib. It comes down to bevacizumab. I can tell you what people don’t say: “I like that bevacizumab/niraparib combination. I didn’t do the bevacizumab with the chemotherapy, but now I am going to do both and maintenance.” You can do that. You can probably even get it [covered by insurance], but it’s not commonly done. If you get maintenance and the patient is not on bevacizumab, people normally use niraparib. Once you start bevacizumab, you don’t want to stop it.

How would you handle this patient now that she has achieved CR with bevacizumab and chemotherapy?
There are some options. If she is on bevacizumab, you can stop it, but [I wouldn’t]. You can say, “I am going to use PARP [inhibition] later.” You’re going to use PARP [inhibition] in the BRCA-mutated population because that’s what works the best or you’re going to stop bevacizumab and give her niraparib. Olaparib [Lynparza] would be off label here, so it wouldn’t [be covered]. Or [you could] add bevacizumab to olaparib, [which is what most would do].
The NCCN [National Comprehensive Cancer Network] guidelines came out in March before [certain] approvals.1 NCCN has the pivot point as bevacizumab—if they’re on bevacizumab and they have a PR [partial response] or CR, they give you the option of bevacizumab plus olaparib.
What is the primary reason you might opt for single-agent bevacizumab over the combination of bevacizumab and olaparib as maintenance therapy for a patient like this?
The patient was HRD positive and taking bevacizumab. The adverse reactions [are a reason not to use the combination]—whatever they might be—or if you tried [the combination] and you noticed it made her too nauseated even when you reduced the dose.
The other thing is that it costs too much for the patient. So, financial toxicity [is another factor] because I think I can manage the adverse reactions; the discontinuation rate of olaparib is only about 10%, so 90% can tolerate it. There are some rare instances when patients can’t tolerate olaparib. But the cost [can be a problem].
The good news is that all of these PARP inhibitors have patient assistance programs. Olaparib’s is called Access 360. The niraparib one is called Together With GSK Oncology. There are websites and 1-800 numbers. We [encourage patients to enroll in] both of those in either scenario because we don’t know… about foundations and co-pays and patient assistance….Olaparib is promoted by 2 [manufacturers], both Merck and AstraZeneca, and niraparib is GSK.
How does the method of administration affect what you use?
I talked about the opportunity of using olaparib and bevacizumab together, but bevacizumab is intravenous. So some people say, “I hear you. In the data, bevacizumab and olaparib make sense, but it’s too tough for my patient to come to the infusion center for bevacizumab, so I am going to give her niraparib.” I get it, but in fact, the manufacturer of niraparib will do a benefits assessment to see if she can get her labs done at home.
And so with the blood pressure monitoring that you can do through telemedicine and the labs done at home, which includes a CA 125 every month, you can overcome that inconvenience with niraparib.
Do you start patients at a higher dose of niraparib and then dose reduce, or do you start lower and then increase the dose?
If a patient weighs less than 77 kg or 170 lb, they should start at 200 mg.2
I start with 300 mg if they’re over 170 lb, and their baseline platelet count has to be over 150,000/µL. You’re right if you start at 200 mg; you only have 1 dose to go to. Most patients that need to get to 100 mg don’t need to discontinue. The discontinuation rates are not any higher for niraparib. In other words, it’s not like they run out of dose reductions. Another reason is because the dose reduction [for niraparib] is steeper; 100 mg is a 50% dose reduction. The dose reduction for the other PARP inhibitors is not quite as steep.
I am not one of those people that starts everyone at 200 mg. I do monitor—I monitor the blood tests weekly on every PARP inhibitor. It’s in the prescribing information for niraparib, but you can have syndromic cytopenias for all the PARP inhibitors. In fact, if I get a platelet count of less than 100,000/µL 1 time, I stop, recover, and dose reduce….We are concerned about thrombocytopenia for niraparib, [so we obtain] weekly platelet counts. If it drops to less than 100,000/µL, stop and dose reduce.
She’s already on bevacizumab, so would you give bevacizumab and olaparib because of the added benefit or would you say a PARP inhibitor is enough?
We don’t have any randomized data to say. I would expect that most of you would add olaparib to bevacizumab. There are some people, including in this poll, who would stop the bevacizumab and pivot to the single-agent PARP inhibitor because of the toxicity issue [and the inconvenience to] come into the clinic. That is a typical answer.
If this patient was homologous recombination proficient (HRP), what would you do?
You would not get bevacizumab plus olaparib paid for [by insurance]. If she’s on bevacizumab and is HRP, and you say, “I am going to try olaparib anyway,” it’s not going to get paid for. And if you in-office expense as I do, it’ll get paid for but it’s going to come out of your paycheck.
How does the use of frontline bevacizumab affect second-line therapy?
I think many of us like bevacizumab [in the second line] after [first-line] bevacizumab. The label is not restrictive after [firstline] bevacizumab, but if you use bevacizumab alone and the patient is PARP naive, we are eager to get a PARP inhibitor in the patient subsequently because, as I said, we want everyone to get a PARP and bevacizumab at some point.
References:
1. NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2020. Accessed November 11, 2020. https://bit.ly/35ZX9PA
2. Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29(8):1784-1792. doi:10.1093/annonc/mdy181