Tafasitamab/Lenalidomide Combo Stands Out for Patients With Relapsed/Refractory DLBCL
During a Targeted Oncology Case Base Peer Perspective event, Ian W. Flinn, MD, PhD, compares the combination of tafasitamab-cxix plus lenalidomide, and selinexor as treatment of relapsed or refractory diffuse large B-cell lymphoma to other other available regimens.
Ian W. Flinn, MD, PhD

During a Targeted Oncology Case Base Peer Perspective event, Ian W. Flinn, MD, PhD, compares the combination of tafasitamab-cxix (Monjuvi) plus lenalidomide (Revlimid), and selinexor (Xpovio) as treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) to other other regimens.
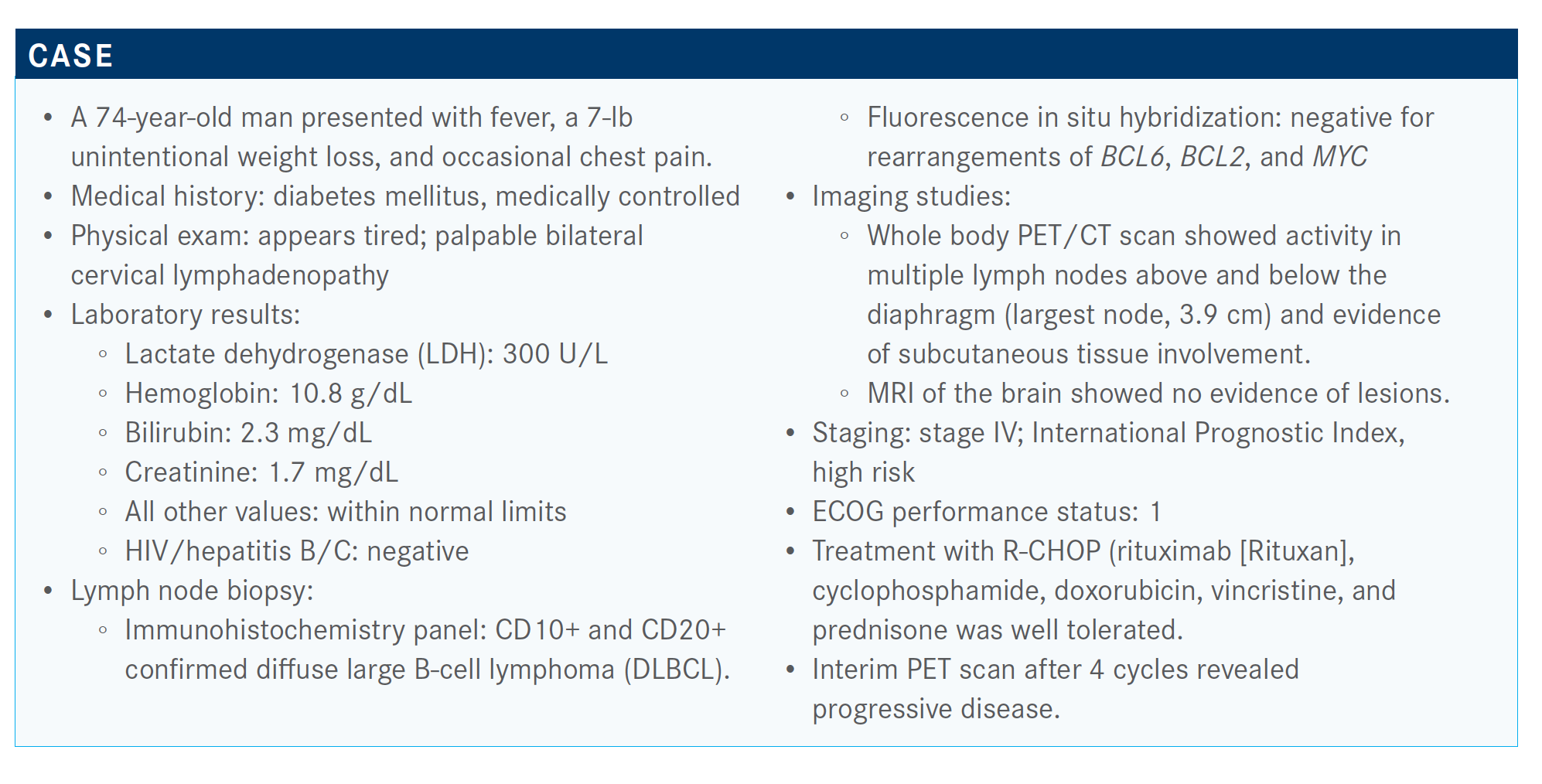
The combination was brought into consideration as treatment of a 74-year-old male patient.
Targeted OncologyTM: What are the most common reasons that patients don’t receive a transplant?
FLINN: From our point of view, the reason that patients don’t [receive] transplant is sometimes about organ function, but it’s really about the disease. It’s the patients that don’t respond to salvage regimen—which is a substantial portion of patients, at least half if not more—who aren’t going to respond [to transplant]. That makes them ineligible, ultimately, even after you [have] referred them. There are some other therapies that we can use in those patients.
What would you most likely recommend for this patient if a clinical trial is not an option?
I think what most of us would use is a salvage chemotherapy in an attempt to get [the] patient to transplant. The issue is that patients who are primary refractory are not likely to respond to a salvage regimen. But we still feel compelled at least to try the salvage regimen or try to get them to CAR [chimeric antigen receptor] T-cell therapy.
What therapy options are there per the National Comprehensive Cancer Network (NCCN) guidelines?
They vary in terms of intensity. They include R-GemOx [rituximab, gemcitabine, and oxaliplatin] and BR-pola [bendamustine, rituximab, and polatuzumab vedotin (Polivy)]. There are some other regimens that might be useful in the second line, as well. I think in subsequent lines of therapy, too, it depends on what the ultimate goal is. You’re still trying to get someone to a transplant. Your choice of regimens might be different if you’re trying to palliate them than if you’re trying to get them to CAR T-cell therapy.1
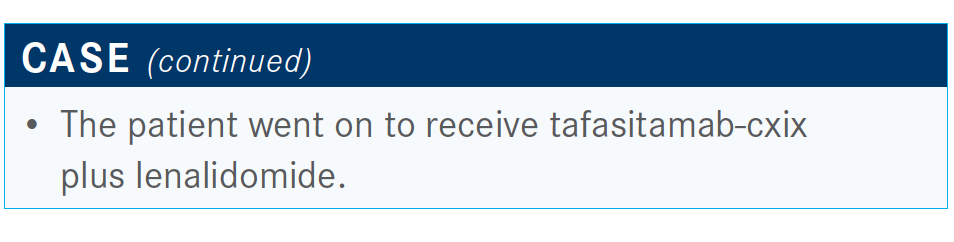
But there are some newer regimens, including the tafasitamabcxix [Monjuvi] regimen with lenalidomide [Revlimid], as well as selinexor [Xpovio].
How is the tafasitamab regimen different from others available in this setting?
One reason is that it has a different mechanism of action. It’s not like giving someone yet another cytotoxic chemotherapy. That’s appealing in the sense that if someone had chemotherapy-refractory disease, perhaps using a different regimen might produce a different outcome for that patient.
Which data support the use of tafasitamab in this patient population?
This summer, tafasitamab was approved in combination with lenalidomide for patients with previously treated relapsed DLBCL.2
The L-MIND study [NCT02399085] looked at the combination of tafasitamab and lenalidomide.3 Basically, tafasitamab is an anti-CD19 antibody that’s been engineered to increase the Fc portion of the antibody [that] has been engineered to improve its ADCC [antibody-dependent cellular cytotoxicity] and ADCP [antibody-dependent cellular phagocytosis] properties. Of course, combining it with lenalidomide makes it a potentially good combination.3
There was an induction schedule, followed by cycles 1 through 3, and then cycles 4 through 12 given every other week. Lenalidomide is given [at] 25 mg daily on days 1 through 21. And then, once you get to a year of therapy, the lenalidomide drops out and patients just receive tafasitamab.
The patients who were eligible for this trial had to have a maximum of 1 to 3 prior regimens. They couldn’t be considered eligible for transplant or have primary refractory disease. The patient we just talked about, in fact, would have been excluded from this trial.
The overall objective response rate was 60% [95% CI, 48%-71%], with many of these patients having complete remissions. Some of these remissions were quite durable, with a duration of response greater than a year [median, 21.7 months]. It did vary, depending on what the patient’s initial response was. Patients who had a complete response, of course, did better than patients with partial responses.
The baseline characteristics of the patients on the trial [revealed] a median age of 72 years. These were predominantly patients with advanced-stage disease, many had elevated LDH, and the median number of prior lines of therapies was 2. About half of these patients had 1 prior line of therapy, and the rest had 2 or more. There were not that many people with primary refractory disease. If they had been primary refractory, they would have gone on to receive another line of therapy before entering onto the trial. About half were refractory to the last treatment.
The progression-free and overall survival [OS] data are impressive. At 1 year, 74% of patients were still alive, and 64% at 18 months. It’s hard to make a direct comparison to other data sets that we already know about. Some people would like to compare this to the [retrospective] SCHOLAR-1 data, which [were from] a study of patients that were refractory to their last treatment or who relapsed within 12 months of a stem cell transplant. In that group of patients, the median OS was about 6 months. This is not that same [type of] patient, but I still think it’s a good outcome compared to what you might expect with, perhaps, immunotherapy.4
What is your reaction to the combination of tafasitamab and lenalidomide?
This trial is hard to interpret in relationship to other studies. The patient population is different. It’s definitely, in my mind, [made up of] fewer heavily pretreated patients. Ultimately, we’ll have to figure out whether these patients would respond.
We know from years of experience trying to get patients who have been primary refractory to respond to R-ICE [rituximab, ifosfamide, carboplatin, and etoposide] that, unfortunately, the response rate is incredibly low. The likelihood of them ultimately benefiting from a transplant is low, as well.
Are there any data on tafasitamab as first-line treatment? For some patients, it appears to be an attractive, less toxic regimen. I’m not aware, but that’s a great advertisement for a clinical trial that’s going to open in the first quarter of 2021, which will be adding this regimen to the R-CHOP regimen. It’s going to be placebo controlled with R-CHOP plus or minus this regimen for patients with large cell lymphoma as frontline therapy. However, I’m not aware of any other use of this combination in the previously untreated patient population. You’d think that in very infirm patients it might be an option when compared with other regimens, and certainly compared with chemotherapy.
What are the toxicity concerns with tafasitamab and lenalidomide?
What you’ll see is that it’s what you would expect from any antibody [combined] with lenalidomide. We’re seeing a lot of [hematologic] toxicities that are common with the R-squared [rituximab and lenalidomide] regimen.
There was not a huge number of unexpected toxicities. Everything is what you would expect. Rash and diarrhea are from lenalidomide. We did a single-agent study a number of years ago with tafasitamab. As a single agent, the toxicity profile wasn’t any different from what you might [expect of] a CD20 antibody.5
Looking at the number of SAEs [serious adverse events] gets to the same point. The number…thought to be treatment related, at least per the investigator, was a fraction of [all SAEs (19% vs 51%)]. In reality, it’s probably the disease that’s causing most of these events.
Who is the ideal candidate for this regimen?
If you asked 10 different [physicians] who specialize in lymphoma, you’d probably get 20 different answers to that question. I think the natural home for this regimen, until we know more about it, is in that group of patients for whom we’re not thinking about cure or who can’t undergo chemotherapy.
There’s early relapse and then late relapse. For late relapse, someone who relapses more than a year after chemotherapy with R-CHOP, the traditional paradigm that we’ve [used] for years is giving salvage chemotherapy with R-ICE and getting [the patient] to transplant. They’re the people that are most likely to benefit from this.
But for these primary refractory or very early–relapse patients, we need to do more.
References:
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 4.2020. Accessed November 10, 2020. https://bit.ly/35H3YW0
2. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed November 10, 2020. https://bit.ly/34Emq2z
3. Salles G, Duell J, Gonz.lez Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/2045(20)30225-4
4. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808. doi:10.1182/blood-2017-03-769620
5. Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29(5):1266-1272. doi:10.1093/annonc/mdy056