Exploring Options for First- and Second-Line Therapies in cSCC
The plan of treatment for a 69-year-old patient with poorly differentiated, infiltrative, cutaneous squamous cell carcinoma was the topic of discussion among Dan Paul Zandberg, MD, and other clinicians in attendance during a Targeted Oncology Case-Based Peer Perspectives event.
Dan Paul Zandberg, MD

The plan of treatment for a 69-year-old patient with poorly differentiated, infiltrative, cutaneous squamous cell carcinoma was the topic of discussion among Dan Paul Zandberg, MD, associate professor of Medicine, director, Head and Neck and Thyroid Cancer Disease Sections, Division of Hematology/Oncology, Department of Medicine, University of Pittsburgh School of Medicine, and Medical Oncology coleader, UPMC Hillman Head and Neck Cancer Program and other clinicians in attendance during a Targeted Oncology Case-Based Peer Perspectives event.
Targeted OncologyTM: What are some noteworthy factors that would affect the prognosis for this patient?
ZANDBERG: Some presentation characteristics to make note of include the anatomic location behind the ear, the numbness that signifies nerve invasion, the size [of the lesion] being 4.5 cm, [and] perineural invasion noted on the pathology report. The lesion appears poorly differentiated, [and there is evidence of] invasion beyond the subcutaneous fat layer. These factors suggest this is a high-risk cSCC.
How do the National Comprehensive Cancer Network (NCCN) guidelines apply to patient’s similar to this one?
The NCCN guidelines provide a breakdown of low-risk and high-risk disease.1 Patients at high risk have lesions in area H and [the lesions are] greater than 2 cm. [Area H] involves the posterior auricular area, as in this patient, as well as the mask area of the face, the chin, the mandible, and also outside of the head and neck. In addition, the genitalia or the hands and feet are high-risk areas, and there are neurologic symptoms such as perineural invasion.
What additional testing would you order?
In this case, the patient has a posterior auricular lesion that seems to be the primary [lesion]. But a good head and neck exam is appropriate to make sure that this is not mucosal [in origin], especially when you’re dealing with other areas of the neck.
It’s important to rule out a mucosal primary. More to the point, if you have a carotid [lesion] and it turns out to be squamous cell carcinoma, there’s a high chance that it’s cutaneous in origin, rather than salivary [in origin]. That is something we see a lot.
[At this point, the tumor suppressor protein] P16 hasn’t been defined as a surrogate for HPV [human papilloma virus] outside of the oropharynx. But I think it’s important to think or make sure this is not a head and neck primary [lesion].
I think we would want some imaging here. We usually like to start with CAT scans in the neck with contrast to evaluate the area and a CT [scan] of the chest. An MRI is an option, but most of us, myself included, like to start with CT to better define with [intravenous] contrast for better characterization.
What factors determine eligibility for resection and/or radiation therapy?
Factors that determine resection ineligibility include involvement of critical structures such as the carotid artery or the base of the skull, especially with tumors that start postauricular. The skin is thin in that area, so any deep diving can quickly get into structures that wind up being around the base of the skull.
The base of the skull is an area where oftentimes full resection is avoided. Prevertebral fascia is another [factor] affecting resectability. And then, extensive perineural invasion is a factor in which the surgeon can’t fully clear the area and leaves disease behind.
One of the major factors that we deal with in the head and neck area is the morbidity associated with surgery. There are times where the lesion can be resected, and it’s all about getting R0 resection, getting negative margins. In the head and neck, especially when you’re talking about near the nose or near the eye you’re talking about a resection that can be very deforming and cause a lot of morbidity.
That always factors into the conversation, especially with elderly patients, in which cSCC occurs often or advanced cSCC [occurs].
How important is engaging your multidisciplinary tumor board when treating these patients?
A multidisciplinary tumor board should review cases [such as] this prospectively and come up with a plan, drawing on all the disciplines present. It’s important to engage our multidisciplinary colleagues for a patient [such as] this relative to evaluation for resection and/or radiation for local treatment options.
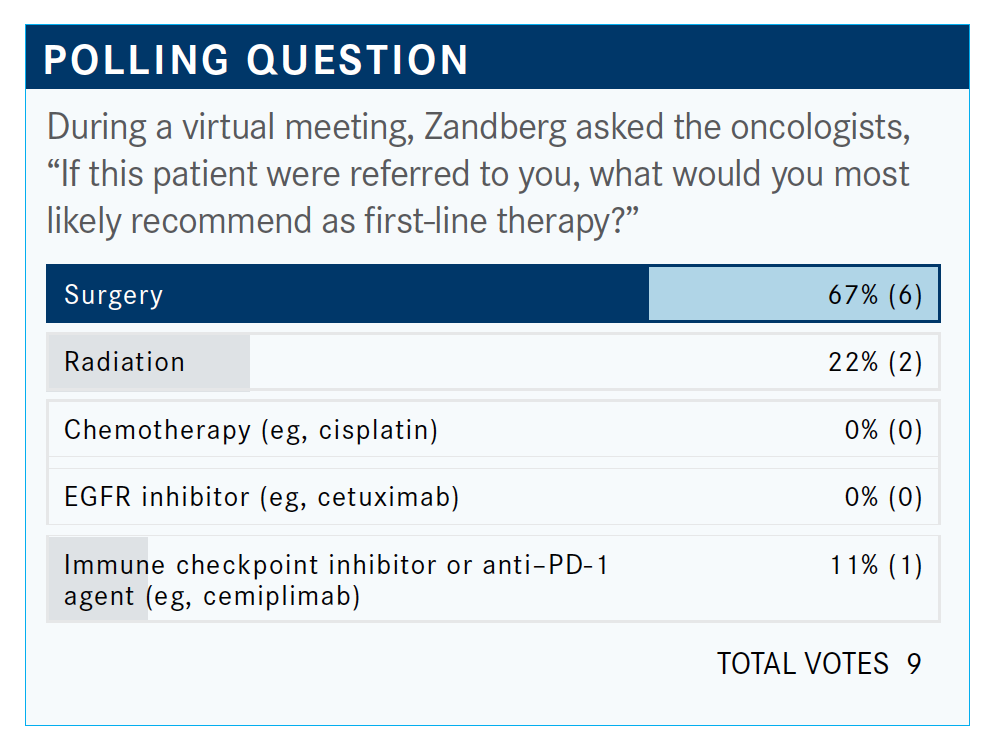
The majority answered surgery and that would be my top choice, as well. The NCCN guidelines also recommend surgery.1 If we don’t have a surgical resection option, we could think about radiation as appropriate. If there are no curative options after surgery or radiation, we would move on to systemic therapy.
What were the key clinical characteristics of the pivotal trial that led to the cemiplimab [Libtayo] approval?
Cemiplimab is approved for advanced cSCC based on the phase 2 trial [NCT02760498].2,3 Patients were stratified into 3 groups, with groups 1 and 2 making up the metastatic cohort. In cSCC, “metastatic” includes patients with regional nodes or locally advanced disease. These patients were treated with cemiplimab 3 mg/kg every 2 weeks for up to 96 weeks. Group 3 received cemiplimab 350 mg every 3 weeks, for up to 54 weeks. This group included patients with metastatic cSCC.
The key inclusion criteria were good performance status and adequate organ function. Patients had to have a target lesion for measurement. You couldn’t have had the option of curative surgery or not [be] amenable to curative surgery. The key characteristic to point out is that investigators excluded anyone with immunosuppression, so patients who underwent organ transplant were excluded, as well as patients with HIV, and some hematologic malignancies such as chronic lymphocytic leukemia where this disease can be enhanced. And these were [patients who were] immuno-oncology naive.
How would you describe the baseline characteristics of the overall cohort?
The patient population represented [patients] that we see in everyday life. This was an older patient population, median age of 72. The majority of patients had cSCC that involved the head and neck, and it was split evenly between locally advanced versus metastatic. [Cemiplimab] is first-line therapy for the majority of patients, as well, with a third of patients receiving prior systemic therapy of some form.
Please describe the response rates.
The investigators reported an impressive response rate of 46% for the total cohort of 193 patients, which is a colossally large trial for cSCC.4
And as seen with immunotherapy, the duration of disease control was long, with a durable disease control rate. Responses were quickly seen, within the first 2 to 3 months. For those [who] are not medical oncologists, most solid tumors have response rates to anti–PD-1 or PD-L1 therapy that range somewhere around 20%, so 46% is very high. In head and neck squamous cell carcinoma, for example, response rates range from 15% to 20% depending on platinum failure or frontline [therapy], so this is a high response rate for an anti–PD-1 [agent] in this disease.
How would you describe the outcomes for this trial?
The investigators observed a median progression-free survival [PFS] of 18 months. At 24 months, the [PFS rate] was 44.2%.
The median [PFS] in the front line with anti–PD-1 is only 4 months, so that shows you the massive difference. Median overall survival was not reached, and a 24-month survival was 73%. Compare that with head and neck squamous cell carcinoma, where only about 10% of patients are alive at 2 years.
Cemiplimab safety was similar to other anti–PD-1 [agents such as] nivolumab [Opdivo], pembrolizumab [Keytruda], and in other solid tumors where these agents have been used with overall good tolerability, including in an older patient population that has advanced cSCC.
If a post hoc analysis was performed, what were the results?
A post hoc analysis evaluated PD-L1 expression [NCT02760498].5 PD-L1 has been predictive of efficacy across solid tumors, albeit not absolute with different cutoffs and different tumor types. This was by tumor proportion score, where basically they found no significant difference. It was numerically higher but showed no significant difference. The point I want to make is that patients responded across PD-L1 status. And PD-L1 is not required. You don’t have to be PD-L1 positive. You don’t have to check PD-L1 status to use cemiplimab for this disease.
Additionally, the investigators looked at tumor mutational burden [TMB]. High TMB is another predictive biomarker for higher likelihood of response. The immune system needs something to recognize, and so mutations in tumor or more mutations in tumors can lead to change in…what we call neoantigen. As a result, [there is] higher likelihood that the immune system may recognize and therefore attack a tumor.
Generally, cSCC has one of the highest rates of TMB of around 61 [mutations per megabase], compared with melanoma, which is somewhere around 20. They did an analysis of this trial and what it’s showing is that people responded regardless of TMB. Maybe a [few] more responded with a higher TMB, as expected, but [there was] not a significant difference based on TMB.
The medians for TMB, or I should say mutations per megabase, [were all] higher than in other solid tumors themselves.
Which immune checkpoint inhibitors are indicated for this disease?
Currently there are 2 agents that are approved for advanced cSCC, [in] disease that’s metastatic or locally advanced when you don’t have a curative surgery and radiation option.
Cemiplimab was the first [approved] and that was in September 2018. Then pembrolizumab was approved in June 2020.6 KEYNOTE-629 [NCT03284424] was a single-arm trial looking at pembrolizumab, and it had similar entry criteria as the cemiplimab trial.7 Investigators observed a response rate of 34% for long duration of response. Median duration of response hasn’t been reached, and this led to the approval of pembrolizumab in this disease.
Is there a difference in response in sun-exposed versus non–sun-exposed areas to these checkpoint inhibitors?
The response rate is around 46%, so not everybody responds. I’ve had [patients] that haven’t responded. I’ve had [patients who] have responded wonderfully. I’ve most recently treated a gentlemen who had refractory disease, and we treated him with multiple rounds of radiation and surgery. And he had a complete response, which is wonderful. He’s doing great. But, to your point about the non–sun-exposed areas, there is a pathology of cSCC from infection or from inflammation.
You know, it’s an open question as to whether those [lesions] in sun-exposed areas do better than those in non–sun-exposed areas. But there are not much data on it. You know, UV [ultraviolet] radiation is thought to be the inducer of the mutation burden or higher level of mutations.
What is the relationship between cSCC and organ transplant?
This is a big interest of mine and a big paradigm for the field. cSCC in the organ transplant setting causes a lot of morbidity and mortality. It’s one of the highest causes of mortality in the transplant patient population, and it results in a 100-fold greater risk of developing a cSCC if [a patient has] undergone organ transplant.
The question is, can we safely deliver these agents?
There [are] probably around 30 case reports looking at patients with not just cSCC but basically any tumor where someone treated a patient with anti–PD-1 agents who had undergone an organ transplant.
A recent paper from The University of Texas MD Anderson Cancer Center [in Houston] looked at their patient experience.8 They had about 40 patients with solid organ transplant that included kidney transplant, which was the most common, and also cardiac and liver. It was pan tumor types, so melanoma, non–small cell lung cancer. Melanoma was the most common, with about 5 patients with cSCC in there. And when they looked at their patient cohort, 41% had rejection overall.
Out of those who experienced rejection, about 81% of patients lost their graft. About 46% died subsequently. And when they looked at anti–PD-1 or anti–CTLA-4 [status], 40% of patients who received anti–PD-1 inhibitors had organ rejection and 36% with anti–CTLA-4 [had an organ rejection].
What’s interesting is that it’s fairly close in terms of risk. between anti–CTLA-4 and anti–PD-1, which is a little different from if you look at all the case reports and especially some earlier case reports, where anti–CTLA-4 seemed to be at lower risk of rejection compared with anti–PD-1.
In this analysis, they looked for patterns. The strongest pattern was that if someone rejected, they rejected quickly. So median time to graft rejection was basically about 2 weeks. What’s interesting is that people responded, meaning we naturally think about immunosuppression as counterintuitive to these checkpoint inhibitors.
We have data now in melanoma and some other tumor types that [show that] if you’re using steroids for immune-related adverse events, it doesn’t affect the efficacy. But these were patients who were being treated with multiple immunosuppressive agents and they were still responsive.
The responses were what you would expect with these tumor types. About 47% had a response. It’s a small patient sample, so I wouldn’t take that as they might respond more. But they do have responses. And they looked potentially responsive. They looked at the outcome by immunosuppressive therapy, and it’s a small cohort. For patients who were on prednisone alone, 78% rejected as opposed to calcineurin inhibitor, such as Prograf [tacrolimus], where only 11% rejected. And those with combined immunosuppressive therapy [have a rejection rate of] 29%.
So, the rejection rate appears to be somewhere around 50% to 60% with anti–PD-1. It’s a paradigm. We know that these patients are at high risk of rejection. With [patients who have had a] kidney transplant, there is the potential for dialysis.
I did have 1 patient I treated [who] lost the graft. I had another patient that I discussed this with—for him [having a kidney rejection] and going back on dialysis was equivalent to death, so he didn’t want to want to go there. I think it highlights the fact that the PD-1/PD-L1 pathway, as well as CTLA4, has a role in normal immunity.
Should the PD-1/PD-L1 pathway be a factor when considering graft immunity?
When you look in the graft, there are immune cells that express PD-L1, and endothelial cells that express PD-L1. There are also PD-1–expressing T cells in there. Investigators took biopsies when rejection started, and about half of the patients in the MD Anderson study who experienced rejection were T-cell mediated.
I think it points to the fact that, as expected, PD-1/PD-L1 pathway interaction is important for graft immunity. I think [this concept] brings up a number of things: How can we better predict who might reject the organ? How can we overcome that? And what else can we do? Can you modulate the immunosuppressive agents in a fashion that’s productive to protect the graft and also still have response?
Are there any trials looking to modulate immunosuppressive agents?
An NCI [National Cancer Institute] trial [NCT03816332] led by Diwakar Davar, [MD], is looking at anti–PD-1 in [patients who have had solid organ transplants] and tumor type with some modulation of the immunosuppressive agents. I should point out that it’s just for [patients who have had kidney transplants]. And we interrogated a tumor microenvironment in immunocompetent versus immunosuppressed cSCC.
We found that those patients who were immunosuppressed, which are patients who have undergone organ transplants, had similar T-cell density as well as PD-1 and PD-L1 expression. We also looked at a cosignaling molecule, B7H3, and we found that it was expressed to a high degree in organ transplant tumor cells, but not on immune cells, including those in the transplant graphs that we have available biopsies on. This is not a new finding; it just means that B7H3 isn’t expressed on immune cells.
The question is, can we go after a cosignaling molecule that’s just expressed on tumor cells? There are agents that block B7H3 or other cosignaling molecules that might be unique enough to spare the graft but help against the immunogenic tumor.
The other question is, are injectable immunotherapies an option for these patients or can they be good options for these [patients who have had organ transplants]?
It’s a big need, and I think for now, we’re left with discussing with patients the risk and benefits.
Have you treated patients with cSCC and myeloproliferative disorders?
I’ve treated some patients who have had some myeloproliferative disorders and they’ve done OK. Nothing to my knowledge contradicts that, but it is a challenge. Obviously, continuing a JAK2 inhibitor is important, but I haven’t had any issues. I have treated 1 patient with myeloproliferative disorder and I haven’t had any issues. There are no data, to my knowledge, to suggest contraindication.
What is your experience with patients who are post stem cell transplant and who also have increased risk of carcinomas of the skin?
That’s a good question. Can’t say I’ve [treated a patient who was] post stem cell transplant. Now, I have treated a patient with head and neck cancer with a cSCC, but I haven’t treated with immunotherapy. It was a patient who had active GVHD [graft-vs-host disease]. There are data for worsening of GVHD. This patient had worsening of GVHD with these immune checkpoint inhibitors.
But I can’t say I’ve had a patient who was post stem cell transplant without GVHD itself. I think it’s a good question. [Patients who have had organ transplants represent] a big need. The question is whether there’s any difference in targets that we can use for this patient population.
Are there any data looking at whole exome sequencing?
We’re looking now at whole exome sequencing, comparing patients who are immunocompetent and immunosuppressed to try to see if there’s anything different in terms of common mutations found in cSCC, which has mostly been immunocompetent analysis itself. We talk about patients who have undergone organ transplant, but patients with HIV are at higher risk of this disease, though not as high as [those who have had an] organ transplant, but higher risk, as are [patients] with autoimmune disorders characterized by chronic inflammation, [such as] Crohn disease.
I’ve treated patients with HIV, quite a few I would say, with different solid tumors. I haven’t had an issue there. Recently, a patient with cSCC that I treated also had Crohn disease and he did OK. There are data out there relative to autoimmune disease and patients not automatically having flares across different tumor types themselves.
After the patient undergoes resection for the cSCC, what do you do next?
There are a number of trials ongoing that are looking at radiation, so there are data on that. But the question is, do we add chemotherapy or not?
The Australian group conducted a randomized phase 3 trial looking at high-risk cSCC post resection [NCT00193895].9 They observed that carboplatin administered weekly with radiation did not improve [freedom from locoregional relapse] compared with radiation alone. There are some caveats to that trial—it was a mix of risk factors, not just some of the traditional risk factors we think of, or at least extrapolate from head and neck, that being positive margins or extranodal extension.
Which combination trials look promising?
One trial that we have open here at Hillman [Cancer Center] is avelumab [Bavencio] plus cetuximab [Erbitux] versus avelumab alone [NCT03944941]; it’s for advanced cSCC. It does include patients who are HIV positive, but not patients who have undergone organ transplant. If you’re on the avelumab alone arm and you progress, you can then have cetuximab added. So it’s going to evaluate the combination of therapy versus avelumab alone and then also simultaneously analyze for those [who] fail immune-oncology, in this case avelumab, the addition or subsequent addition of cetuximab, and whether that will boost efficacy.
References:
1. NCCN. Clinical Practice Guidelines in Oncology. Squamous cell skin cancer, version 2.2020. July 14, 2020. Accessed November 12, 2020. https://bit.ly/3f0m9dO
2. FDA approves cemiplimab-rwlc for metastatic or locally advanced cutaneous squamous cell carcinoma. FDA. September 28, 2018. Updated January 18, 2019. Accessed November 13, 2020. https://bit.ly/32BXQ0L
3. Migden MR, Khushalani NI, Chang ALS, et al. Primary analysis of phase 2 results of cemiplimab, a human monoclonal anti–PD-1, in patients (pts) with locally advanced cutaneous squamous cell carcinoma (laCSCC). J Clin Oncol. 2019;37(suppl 15):6015. doi:10.1200/JCO.2019.37.15_suppl.6015.
4. Rischin D, Khushalani NI, Schmults CD, et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): longer follow-up. J Clin Oncol. 2020;38(suppl 15):10018. doi:10.1200/ JCO.2020.38.15_suppl.10018
5. Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294-305. doi:10.1016/S1470-2045(19)30728-4
6. FDA approves pembrolizumab for cutaneous squamous cell carcinoma. FDA. June 24, 2020. Accessed November 13, 2020. https://bit.ly/3f3O3pi
7. Grob JJ, Gonzalez R, Basset-Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II trial (KEYNOTE-629). J Clin Oncol. 2020;38(25):2916-2925. doi:10.1200/JCO.19.03054
8. Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7(1):106. doi:10.1186/s40425-019-0585-1