New Evidence Supports Targeted Therapies in Early-Stage NSCLC With EGFR Mutations
During a Targeted Oncology Case Based Peer Perspectives event Karen L. Kelly, MD, discussed molecular testing guidelines and testing execution for early-stage non–small cell lung cancer as well as the research supporting the use of targeted therapies for treatment.
Karen L. Kelly, MD

During a Targeted Oncology Case Based Peer Perspectives event Karen L. Kelly, MD, professor, Hematology and Oncology, Jennifer Rene Harmon Tegley and Elizabeth Erica Harmon Endowed Chair in Cancer Clinical Research, University of California, Davis associate director for Clinical Research, UC Davis Comprehensive Cancer Center, discuss molecular testing guidelines and testing execution for early-stage non–small cell lung cancer (NSCLC) as well as the research supporting the use fo targeted therapies for treatment.
Targeted Oncology™: Who on the multidisciplinary team orders molecular testing?
KELLY: That is a big controversy. There are many places that do pathology reflex testing. We went back and forth about that. We like to do it ourselves because when you have that patient in front of you, and you have limited tissue, we want to prioritize exactly what [tests] we want. If they’re a current smoker with a 10-pack-year history, we may want to test for PD-L1 expression and have the NGS [next-generation sequencing] as the secondary test. We like to be able to control that when the tissue is insufficient.
At disease recurrence, can you still use information from an initial biopsy, or do you need to rebiopsy because mutations could evolve over time?
The driver mutations don’t evolve over time. If they have an EGFR mutation right now and the patient relapses in 2, 3, or 4 years, you are still going to see that EGFR driver mutation. Remember, those are clonal mutations. Those mutations are always there. Now, what can evolve are the partners. Maybe you didn’t have a TP53 mutation before, but now you do. Those things are evolving, but the fundamental driver of the tumor does not evolve. You can never say never, but [that is the case] for the most part.
You’re good once you know they have an EGFR mutation. It’s always wise to biopsy again when they’re that far out; and certainly, you could get NGS. But you wouldn’t absolutely need to. You could do it for academic interests, but they should still have that same EGFR mutation or whatever oncogenic driver mutation they had in the beginning [of treatment]. That’s the point about knowing up front what they have. I think for some patients, they’re comforted by the fact that if they do progress, you now know exactly what plan B is. Patients are going to get osimertinib [Tagrisso] if they have stage IV disease, and I think that’s reassuring to patients.
Can they shift to oncogene positivity later on, as we see in breast cancer with HER2 positivity?
No, it would be rare for that to happen. Either you missed it when you sent the NGS or you missed it in the sample that was sent. That could happen. I think that would be rare, though. When tests come back as negative [for the gene], it doesn’t hurt to repeat them. But once it’s positive, you don’t need to repeat it again because it’s not going to evolve. At least as of today, we don’t have evidence of that. But if things are negative, it certainly wouldn’t hurt to repeat that.
The other thing is once the patient has tested positive on NGS by tissue and they have stage IV disease, you know a liquid biopsy should confirm that they have that same EGFR mutation.
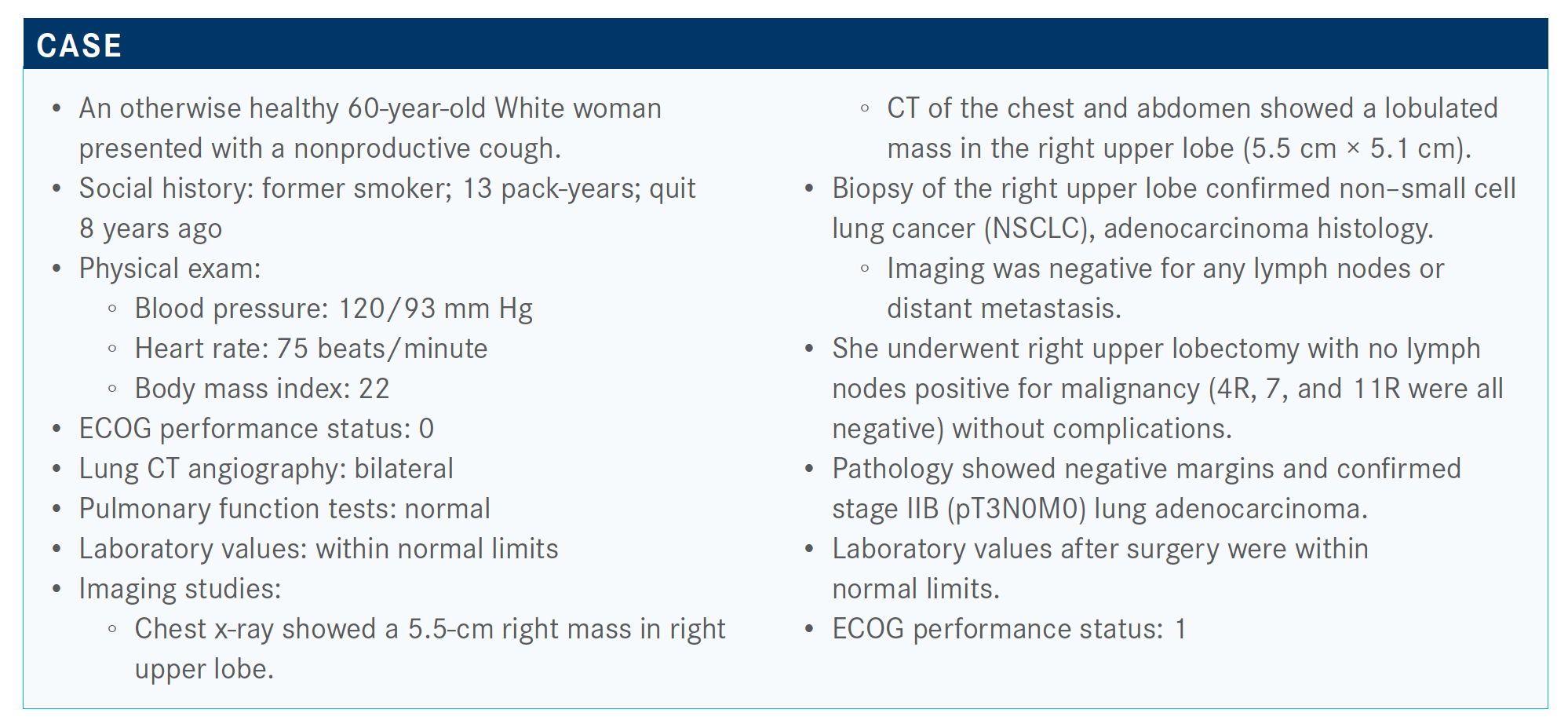
What do you think of the poll results?
What I’m seeing coming across is that most patients are receiving adjuvant chemotherapy. It’s not 100%, of course. What I see are my elderly patients—typically, they’re 80 years old—that I sometimes have a hard time convincing. Even though they are 80, they can still be in good shape, but they’re so concerned about the toxicities that they don’t want to have chemotherapy. Even when I try to say, “We can do a dose modification,” and things [such as] that, it’s the group of patients that I struggle with.
How long do you wait to give the chemotherapy following surgery?
My target is 6 weeks, but I have done 3 months. I’ve even done 4 months, but I do put a hard stop on myself at 4 months. Sometimes, patients take a bit longer to recover.
We’re all pretty clear that most patients should have adjuvant chemotherapy. This patient did have a large tumor; it was 5 cm. The data support [adjuvant chemotherapy in] tumors that are greater than 4 cm. I don’t think there’s any question about end status from all of the adjuvant trials. The patients that do the best are the patients who have N2 disease, then N1, and then the N0.
But the question one would ask is about those patients who have that 3- to 4-cm tumor, patients who have “poor risk” features [such as] a lymphovascular invasion or poorly differentiated, visceral pleural involvement. We don’t have a lot of good data here, but we have some case reports. There was a study out of Japan—but that was even in tumors that were smaller, less than 2 cm—and it did appear in their algorithm [that] those patients who had these types of features did [worse]; but if you gave them adjuvant chemotherapy, they did better.1 I don’t think I’ve ever given adjuvant chemotherapy to somebody with a 2-cm tumor, but I’ve probably done it [for tumors] between 3 and 4 cm.
I get nervous, particularly when I see things [such as] vascular invasion. With that, you know you are set up for metastases. Age is really a prognostic feature.
What do the guidelines from leading cancer organizations have to say about the issue of giving adjuvant chemotherapy in resectable disease?
[In general, they indicate] no adjuvant therapy for the stage IA and IB, which is that 3- to 4-cm tumor. Then, everybody else gets adjuvant chemotherapy.2
What parameters are important in deciding whether to administer radiation therapy?
Radiation therapy has now changed as of the ESMO [European Society for Medical Oncology] Congress. Typically, this is a great question that comes up in our multidisciplinary tumor boards. For those patients who have an R0 resection and they had the microscopic N2 disease, do you give radiation therapy? I think the dogma before the ESMO meeting was to answer yes, that we would often report to those patients and refer them to radiation therapy. At least, that’s our tumor board recommendation for N2 disease, not for N1. But for N2 disease, they [received] neoadjuvant chemotherapy. They still had N2 disease at resection or they didn’t get any chemotherapy, and they’re now stage III. We would send them for radiation therapy after they finished their adjuvant chemotherapy.
An incredibly simple trial conducted by our European colleagues asked a simple question, and that was “Does PORT, postoperative radiation therapy, for N2 disease improve diseasefree survival [DFS]?” It comes up every week at our tumor boards. These results were presented; it was called the Lung ART trial [NCT00410683], and there was no benefit to PORT.3 The other thing that I will tell you is that the patients had more toxicity, as well. I’m not a radiation oncologist, but there were intriguing toxicities that occurred on this trial for those patients [who received] PORT. I just wanted to point this out if you didn’t have an opportunity to see those results. This is practice changing. PORT will no longer be offered to patients with N2 disease.
What do we know about EGFR-targeted therapies in the adjuvant setting that could be applied to this patient?
The right answer would be to enroll this patient into the ALCHEMIST trial [NCT02194738] at this point. ALCHEMIST was based upon data that came from 3 trials.
The RADIANT trial [NCT00373425], after adjuvant chemotherapy, looked at erlotinib [Tarceva] versus placebo. When we looked at a subset of patients who had the mutation, and this was a small number of patients [who] had the mutation, those who received erlotinib did have improved DFS. But over time, the Kaplan-Meier curves for DFS meet. This was a subset analysis. It wasn’t powered for statistical significance.4
Then there was a study done at Stanford by Joel W. Neal, MD, PhD, and colleagues, looking at giving erlotinib [NCT00567359]. This was a single-arm institutional trial. When you put all the patients together, it did look better than the historical control. There are 10 of these 100 patients [who] had stage IA disease on this trial. Those patients would automatically do better.5
Then there’s the ADJUVANT trial [NCT01405079] coming from our colleagues in China. The trial was looking at gefitinib [Iressa] versus chemotherapy, not as maintenance but as the treatment. This trial did show a DFS benefit, but as everyone heard at the ASCO [American Society of Clinical Oncology] Annual Meeting this year, there’s no OS [overall survival] benefit. This trial is not powered for [OS]. But this is an important question about whether we’re just extending disease, delaying recurrence, and if that effective treatment in stage IV disease is as valuable.6
Which data support the use of osimertinib for this patient?
That leads us now to the ADAURA trial [NCT02511106]. In the lung cancer world, this was the most controversial abstract presented at the ASCO Annual Meeting. It was so unfortunate that we weren’t there in person to debate it among ourselves after the presentation.
This was a trial that looked at osimertinib in resected early-stage disease. Patients were randomized to receive osimertinib 80 mg or placebo for 3 years. That’s a bit different from what the other trials were doing, which was 2 years. The other important thing here is that the primary end point was DFS only in the stage II and IIIA groups. That was the primary end point because those patients were the patients that one would expect to do the best. It was not [powered for] OS.
What were some of the issues surrounding this trial that made it controversial?
The first controversy here is about [whether this] should have been an OS end point in an early-stage disease study. That is the gold standard, and there’s no question about it.
One point that led to DFS being the end point [is that] we can get to it a lot faster. Is it truly a surrogate for OS? Now, I will say that with chemotherapy, if you looked at all of the adjuvant chemotherapy trials, DFS clearly was the surrogate. There were no trials in the adjuvant setting where if DFS was positive, then OS wasn’t positive. The confounding variable here is the fact that osimertinib is so effective in the stage IV setting. It may not result in an OS benefit because the drug is so effective in the stage IV setting.
That’s where there [are] a lot of controversies. This was the most controversial topic, OS versus DFS. Most trials today in the neoadjuvant setting have looked at a DFS end point. DFS has become a surrogate marker, and the FDA has recognized that. I think that there’s still value in DFS. Yes, we absolutely want to cure more patients, but having people live longer is still important. Never getting to stage IV disease is also incredibly important.
What were the patient characteristics of those treated on ADAURA?
The only thing that was controversial here—remember, this is an international trial—is the fact of adjuvant chemotherapy. There were 55% of patients [who received] adjuvant chemotherapy, but 45% of patients did not get adjuvant chemotherapy. Now, it was well balanced [between arms], but this was surprising. We just said the majority of our patients [receive] adjuvant chemotherapy; but internationally, that just isn’t true. There are many reasons why that happens, and I’m not going to belabor it, but we still need to take a look into that. That was surprising, that the number of patients who [received] adjuvant chemotherapy was only 55% in each one of these arms.
Of course, you could say it’s the stage I patients [who did not receive adjuvant chemotherapy]. This study was recently published in the New England Journal of Medicine, and that’s what they said in the publication. That the patients with stage II and III, the majority of them did [receive] adjuvant chemotherapy.
What was the outcome for the primary end point of DFS?
The primary end point in stage II and IIIA disease is highly impressive. There’s no doubt about that, with the hazard ratio of 0.17 [95% CI, 0.12-0.23; P <.0001].7 This trial was closed early by the data safety monitoring committee at an unplanned interim analysis.
This was unprecedented. The data safety monitoring committee’s focus was on safety in this trial, but because they had seen some of the early results, they requested more information. The statistical assumption here was for a hazard ratio of 0.70, and this hazard ratio was 0.17. In early-stage disease, we did not expect this significant of a hazard ratio. Because of that, the independent data monitoring board recommended that we unblind the trial.
One of the stipulations [made] by the committee was that we keep the patients themselves and physicians blinded so that we could preserve that OS end point because we knew it was going to be controversial. People think the trial is completely unblinded to the patients and the doctors, but it is not. The other thing that has been done on this trial is that for patients who progress on the placebo arm, they will cross over to osimertinib on the trial. That is the right thing for the company to do.
What were the outcomes for the secondary end point in this trial?
Not only did they see a DFS benefit in the stage II and III disease setting, but [it was seen] even [in] those patients with stage IB disease. These are [the patients who have] the greater-than-4-cm tumor. Those patients [saw a hazard ratio of] 0.21. It’s not quite as impressive, but still incredibly close. Because this trial was stopped earlier, the median follow-up here is only about 2 years. It is immature data, so we have to keep that in mind. But for these [Kaplan-Meier DFS] curves to ever come together, that would be not statistically significant. It is not probable, even with longer follow-up. We will see, but there was never going to be a way for these curves to ever come together and be nonsignificant.
The benefit was seen across all patient subsets. Even those patients [who] did not get adjuvant chemotherapy still benefited [HR, 0.23; 95% CI, 0.13-0.40]. There’s no confidence interval line approaching 1; all patients did benefit.
By disease stage, the curves don’t look as impressive when you look at just the [stage] IB tumors, but the hazard ratio is still 0.5 [95% CI, 0.25-0.96]. It’s still statistically significant.
Is this a reasonable option for patients with central nervous system (CNS) metastases?
When I think about osimertinib, I think about it as a trifecta. I think about overall efficacy, efficacy in the brain, and that’s what we’re seeing here. One of the added values here that were reported at ESMO and in the paper is a reduction in CNS disease. From being the principal investigator on the RADIANT trial, I’ve seen that about 37% of the patients on that study developed isolated brain metastases. This is a real problem for patients who have EGFR-mutated lung cancer as well as ALK and other oncogenes. The ability to delay or prevent brain metastases—I find this highly valuable. Nobody wants brain metastases. This showed a stellar result, with a hazard ratio of 0.18 [95% CI, 0.10-0.33] in patients with CNS disease.
Is there indication of a survival benefit with adjuvant osimertinib?
In the ASCO presentation, they did show a survival curve, but it’s premature. I don’t want to make anything of it because they're premature data. But it’s headed in the right direction. The P value here is .04, but I can’t believe that there would be any scenario where the osimertinib curve would fall below the placebo curve in any significant manner. I could be proven wrong, but I don’t think so.
Were there any new safety signals with osimertinib in ADAURA?
It has a mild toxicity profile. Osimertinib is so well tolerated, it’s impressive compared with even dovitinib. With osimertinib, patients had rash, diarrhea, but we just don’t see a lot of toxicity. The grade 3 toxicity rate was 10% for the osimertinib versus 3% for placebo. Any-grade adverse effects are at 90% versus 55%, but overall, the drug is incredibly well tolerated. It’s efficacious in the brain with mild toxicity. This is why this drug is truly the gold standard.
Osimertinib is the icon of all TKIs [tyrosine kinase inhibitors] and what every other TKI wants to be able to do. And many do, although, I have to say the toxicity profile of osimertinib is the best I’ve seen of any TKI, whether it’s for targeting ALK, MET, or others.
References:
1. Yoshino I, Yoshida S, Miyaoka E, et al; Japanese Joint Committee of Lung Cancer Registration. Surgical outcome of stage IIIA- cN2/pN2 non-small-cell lung cancer patients in Japanese lung cancer registry study in 2004. J Thorac Oncol. 2012;7(5):850-855. doi:10.1097/JTO.0b013e31824c945b
2. Spigel DR. Discussion of LBA5. Presented at: American Society of Clinical Oncology 2020 Virtual Scientific Program; June 1-4, 2020. Accessed November 5, 2020. https://bit.ly/38gIEd8
3. Le Pechoux C, Pourel N, Barlesi F, et al. An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann Oncol. 2020;31(suppl 4):S1178. doi:10.1016/j.annonc.2020.08.2280
4. Kelly K, Altorki NK, Eberhardt WEE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol. 2015;33(34):4007-4014. doi:10.1200/JCO.2015.61.8918
5. Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(2):97-104. doi:10.1200/JCO.18.00131
6. Zhong WZ, Wang Q, Mao WM, et al; ADJUVANT investigators. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139-148. doi:10.1016/S1470-2045(17)30729-5
7. Wu YL, Tsuboi M, He J, et al; ADAURA investigators. Osimertinib in resected EGFR mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071