Roundtable Discussion: Kuzel Walks Through New Treatment Options in Advanced RCC
During a Targeted Oncology case-based roundtable event, Timothy Kuzel, MD, discussed risk assessment and later-line treatment options for a patient diagnosed with metastatic renal cell carcinoma who received lenvatinib and pembrolizumab.
Timothy Kuzel, MD
Samuel G Taylor III MD Professor of Oncology
Professor, Department of Internal Medicine
Division of Hematology, Oncology and Cell Therapy
Rush Medical College
Chief, Division of Hematology, Oncology and Cell Therapy
Interim Deputy Director, Rush Cancer Center
Chicago, IL


KUZEL: How do [clinicians] assess patient risk nowadays? Do you go through the steps involved [for calculating the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium) risk score]? Or use any of the various algorithms? Or do we just sort of look at the stage and leave it at that?
GUNDALA: Yes, we do look at the score. And we take into consideration all the risk factors that are mentioned on the [IMDC] risk calculator: the duration from the surgery to reoccurrence, patient performance status, and all the laboratory values—calcium, hemoglobin. That helps you separate the patients [with] intermediate or poor prognosis vs favorable [prognosis].
KUZEL: [The IMDC] has been updated recently, so once you make that decision, do you use different therapies for favorable, intermediate, poor [prognoses]? Or at this point does it matter?
GUNDALA: We have so many options that I don’t know if there is a slight difference between them, between the favorable and less favorable [prognoses]. Because we can use the pembrolizumab immunotherapy combination with lenvatinib [or axitinib (Inlyta)]. Of course, we have the doublet immunotherapy. but I’m not clear if there is one better than the other, besides the data that I know about nivolumab [Opdivo] plus ipilimumab [Yervoy].
SHAH: I do have a couple of patients in later lines of therapy [who have] failed all other therapies and appreciate your option always. And I think there is something special about…nivolumab plus ipilimumab and I’ve had patients [with intermediate and poor risk] respond to that combination….I think [for patients at] intermediate or poor risk I’m still using nivolumab plus ipilimumab. Favorable risk, certainly pembrolizumab plus lenvatinib or pembrolizumab plus axitinib is reasonable.
SHEKHANI: The rationale for separating intermediate vs low risk vs high risk is because you can choose a treatment based on the risk….You don’t want to use high-risk medications for low-risk patients who can benefit from a single-agent pazopanib [Votrient] or some “nib,” rather than combination immunotherapy.
They do carry high risk, so I personally separate them out based on the risk factors. Just so that I can choose and appropriately [assess the] risk [of the] treatment for the disease that I’m dealing with.
KUZEL: I’m glad you mentioned some of that. At least some of you still are using single-agent, tyrosine kinase inhibitors [TKIs]? You don’t automatically default to one of the doublets?
SHEKHANI: Not in a low-risk patient, not in a high comorbid patient, not in an elderly patient with high risk, or a patient with autoimmune disease. I would prefer to use a single-agent “nib.” [And not] if the patient is at low risk and if the treatment carries a higher risk than the disease itself does.
SHULMAN: If they have very slow-growing disease, I’ve just done a single agent as Dr Shekani mentioned—just as a trial to see how they would do with that before putting them on 2 different drugs. If they have very small, indolent disease...or as in this case with the calcium being marginally elevated, it might make me a little more nervous about observing them.
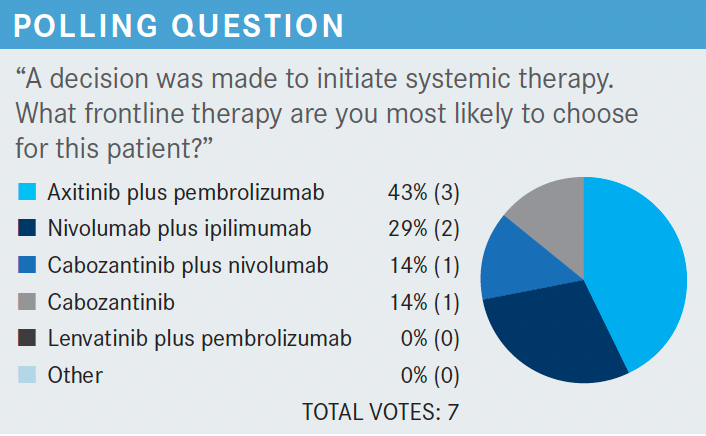
KUZEL: Does anybody have experience with lenvatinib in any setting either with everolimus [Afinitor] or pembrolizumab? Does anybody use that for their relapsed-refractory patients?
TAJUDDIN: I’ve used it in [treating patients with] thyroid cancer.
KUZEL: OK, so you have lenvatinib experience in a different disease, but at least you have that experience. That’s the beauty of talking to [clinicians] who do a little bit of everything. They may have used some of these drugs in other disease settings. Has anyone ever used it in kidney cancer with everolimus?
GUNDALA: Yes, I did use it.
KUZEL: What did you think?
GUNDALA: It wasn’t easy. [For] the stomatitis we needed the dexamethasone and we knew what to do about that, but the hypertension and edema, proteinuria, asthenia, those are difficult issues that we needed to dose down the patient. [The patient] did respond, he had a significant response and duration of response, but it was not easy in terms of quality of life and [adverse] effects.
KUZEL: Which drugs did you blame for the toxicities mostly?
GUNDALA: I think initially I dosed down the everolimus. And then subsequently I had to dose down the lenvatinib, I think, because the edema was getting worse.
KUZEL: Nobody has used it with pembrolizumab, I’m guessing.
SHEKHANI: I am currently using [it] on 1 patient, pembrolizumab with lenvatinib for anaplastic thyroid carcinoma. It’s based on a single study—it’s a phase 1, early phase 2 [trial] from [The University of Texas MD Anderson Cancer Center in Houston]. And after discussing with MD Anderson, we decided to try that. Luckily we were able to get approval as well. And so far the patient has been tolerating it fairly well. But it’s a combination for anaplastic thyroid.
KUZEL: Right. But, at least in terms of the combination, you haven’t run into any unusual toxicities that would have made it easier or more difficult to treat?
SHEKHANI: No, in this particular patient, I am finding lenvatinib to be better tolerated than I found in a younger man with metastatic thyroid carcinoma when it was single agent, and I had a difficult time getting that one in that [patient]. So I was just dreading it, [worrying] that it was going to be a problem, especially with the pembrolizumab diarrhea, the lenvatinib diarrhea, the stomatitis, and all those things. But this patient has done amazingly well.
KUZEL: What [do you] think of the CLEAR [NCT02811861] data?1
SHULMAN: I think one thing that struck me is there’s a big difference in progression-free survival between the pembrolizumab plus lenvatinib [group] vs [the group receiving] sunitinib [Sutent].1 But the survival data, and again it’s a shorter follow-up, but the curves kind of come together and you might have a more effective combination inducing a response or shrinking disease. But then you wonder if you look at the composite of complete or partial response, or stable disease if they are more similar in that vein.

KUZEL: So, do you think about second-line or-third line therapy when you’re making a choice for first-line treatment? Or do you avoid a drug that would be in a combination in the frontline setting because you want to use it in the second-line setting?
TAJUDDIN: I think with advanced and high-risk metastatic disease you have the window of opportunity to treat ahead the first time. You might never get a second opportunity, so might as well use the best you have.
SHEKHANI: Same here—first try to give it your best shot. You don’t know if you’re going to have a good enough performance status to go with a more aggressive second line or not. [Also,] with the precision medicine you can identify some of those medications based on that as well.
So it’s not necessarily going to be less “pick from the basket” and see which one comes out. It’s [determined by whether] the precision medicine gives you some idea [to] target on either first line or second line.
SHEKHANI: I did 14 mg, 20 mg, and 24 mg. I think 24 mg is the highest one that’s been used in any trial—any single agent as well. But 12 mg and 14 mg are probably [used often]; 14 mg I think is the one they use more commonly.
KUZEL: Lenvatinib at 18 mg and 5 mg of the everolimus are the starting doses.2,3 In the CLEAR trail, the lenvatinib with pembrolizumab is actually 20 mg.1
TAJUDDIN: For thyroid [cancer], the recommendations are the escalating doses of lenvatinib. Is something such as that useful here?
KUZEL: You know, that’s an interesting question. There are [clinicians] who have been proponents that for some of these toxic combinations maybe we should start lower, and if they tolerate it, escalate. Then, there are others who feel that if you do that you don’t get a response; you’re going to bail out when it had just started at the higher dose.
So with lenvatinib, which I tend to use it in salvage, sometimes I don’t start at 18 mg because I just know based on prior TKI experiences with that patient they will never tolerate 18 mg. So I’ll purposely start lower, but with lenvatinib plus pembrolizumab in the frontline setting. I don’t think starting low makes a lot of sense. I think you go with full dose and deescalate if the patient doesn’t tolerate it and I think that makes the most sense in the frontline setting.
The dosing of lenvatinib is 20 mg daily, and the pembrolizumab was given every 3 weeks. I’m sure many of us probably would give it every 6 weeks, but in the trial it was given every 3 weeks [at] the older dosing.1 And the pembrolizumab was given for 2 years. At the end of 2 years investigators were allowed to continue lenvatinib as a single agent for as long as the patient tolerated it, or [until] the patient progressed, or [if] the patient just stopped wanting to take it. So that’s a little bit different than some of the combinations where you might give it for just 2 years.
The dose forms of lenvatinib, which I mentioned, are either 4-mg or 10-mg capsules.2 When you start with lenvatinib it’s 18 mg, so it’s a 10-mg capsule and 2 of the 4-mg capsules. In this case you start with two 10 mg capsules, so the next dose reduction down is the 14-mg dose. That’s your sort of deescalation scale. I’ve never given anybody 8 mg but I dose reduced a lot of patients down to 10 mg with that drug.

KUZEL: Do you like this CheckMate 9ER regimen [of nivolumab plus cabozantinib (Cabometyx)]?4 Have you tried this? Has anybody used it in the salvage setting?
SUH: I’ve used this regimen in a few patients with metastatic disease. And the reason I chose it is that I have some experience with cabozantinib and with immunotherapies. [It is] well tolerated so I felt comfortable doing this combination.
KUZEL: Are there certain patients that you would skew toward this regimen over pembrolizumab plus axitinib? You have 2 patients sitting in your office. Would you look at [one of them] and say I’m going to do cabozantinib plus nivolumab for this one for some reason?
SUH: For my patients they were both intermediate for risk. Because of the more recency of the data, I wanted to gain more experience in using this regimen.
KUZEL: If a patient has bone metastases, does that factor into anybody’s thinking? You might say, “I heard something about bone metastases with cabozantinib, maybe I’ll give nivolumab plus cabozantinib a try.” Or hasn’t that penetrated?
SHULMAN: I just heard that bone metastases are difficult with immunotherapy but it wasn’t that cabozantinib was better.
KUZEL: Would you even give a combination? Or would you shy away from a combination?
SHULMAN: Well, it seems from what you’re saying that you don’t know if there’s synergy. With cabozantinib plus nivolumab, it may add something to the cabozantinib.
KUZEL: So do you think that these are just additive combinations?
SHULMAN: I think the response rates indicate maybe there’s some synergy and it seems [as though] the progression-free survival may be additive, but it seems you’re getting a lot more response than you would with sequential therapy.

KUZEL: Is the goal when we treat these patients to just extend progression-free survival by a few months? Or is cure what we’re shooting for? When you counsel a patient with kidney cancer, what do you tell them?
SHULMAN: Unless they’re getting high-dose IL-2, I try to give them the best response, with the best quality of life, and help them live the longest. In general their disease is not going to be—hate to use the word cure—not curable, but that’s kind of how I say it. It’s not as though you’re going to die from it, but we can’t cure it.
KUZEL: I use the C word [cure], and I certainly use it with ipilimumab plus nivolumab and I think the justification for these is similar. The TKI is supposed to make the nivolumab work better or the pembrolizumab work better, so it’s not necessarily just additive; hopefully it is synergistic.
And it’s the tail on the curve that we’re obviously all interested in and with only 2-, 3-, 4-year follow-up, we’ve done so well that all the patients are still alive. We haven’t even reached the median survival yet. I think it will be interesting to see if there’s a tail on that curve with these combinations. I think hopefully there will be.
TAJUDDIN: So those who survive longer, is that the nature of the disease that they have? That’s why they’re surviving longer? Or is it just their therapeutic interventions that are changing and [helping] them live longer?
KUZEL: Well, that’s why the risk group stratification is important, I think, and the makeup of the trials is important. We know that intermediate- or poor-risk patients have actually relatively short survival. I mean, they have disease and [those with] favorable risk have a long survival. When you put a lot of favorable-risk patients [in a trial] it clouds that picture, but intermediate- and poor-risk patients don’t do very well. I think that if you can [affect] the natural history on intermediate- and poor-risk patients, it’s treatment related.
[I had a patient where] we took his lung mass out and everything went away, spontaneous remission. It is always hard because they were always so infrequent, but with these regimens the response rates are very high and hopefully durable.
KUHL: Does anybody think that the tolerability of the nivolumab plus cabozantinib looks better than their pembrolizumab plus axitinib experience? Or better than the lenvatinib plus everolimus or the lenvatinib plus pembrolizumab data? Was there a toxicity reason to choose maybe one of these regimens [over another]?
SUH: I found the lenvatinib is quite difficult for patients to tolerate, I’ve used it in patients with thyroid cancer.
There are a lot of issues with weight loss, anorexia, and [gastrointestinal] toxicities. I prefer cabozantinib over lenvatinib.
KUZEL: So the TKI toxicity does help you choose your regimen. Do you like to use axitinib? I mean, nobody used to like using sunitinib. Is axitinib a preferred TKI? Do you find you’re comfortable with that in terms of managing toxicities?
SHEKHANI: [There are] no data, but I would prefer poziotinib, over axitinib in time. Because I think my experience with poziotinib has always been the best. But axitinib is OK. Unfortunately, hypertension has been a problem, cardiac issues have been a problem [for] those patients on axitinib.
KUZEL: Of course, single-agent cabozantinib has been a choice in this group of patients. [In terms of doses of] 60 mg of cabozantinib vs 40 mg of cabozantinib, do you find a big difference? Or do most [clinicians] start at 40 mg when they’re using a single agent of cabozantinib?
SHEKHANI: [Yes] 40 mg, I could count the number of patients on my fingertips who have been able to tolerate 60 mg. The 40 mg has been better. The 20 mg—most of them are usually able to do that.
KUZEL: You don’t start at 60 mg and rapidly dose reduce? You have given up on 60 mg?
SHEKHANI: [It] depends on who’s on call on the weekend when I first start. If I’m on call, I’m starting [at] 40 mg.
KUZEL: [If you have] patients sitting in front of you, is there any clinical feature of a patient that makes you choose one regimen over the other? Or are most of you [using a] favorite regimen and you’re going to stick with it most of the time?
SHEKHANI: For me, if it’s mostly bone lesions, I prefer not to have dual immunotherapy or try to have at least one of the “nibs”. But, if it’s a visceral disease, and I need a quick response, and there’s no other comorbidities or anything like that, I may go for combination immunotherapy.
KUZEL: OK, what about brain metastases? Do they factor in at all when you’re thinking about these regimens?
SHEKHANI: For the brain metastases I prefer to have an immunotherapy, especially combination if the brain metastases are more [of a] priority than any visceral involvement.
KUZEL: Who helps you manage toxicities? Does everybody have nurse practitioners, physician assistants who chip in? PharmDs who work with you? Or does it all just roll back to you?
SHULMAN: Rolls back to us usually. At least in my case.
SHEKHANI: Things were slightly better before the COVID-19 cost-cutting era. But since then it’s been mostly [up to] us.
KUZEL: Is that just because you don’t have enough staff [due to the pandemic]?
SHEKHANI: [Many of] the staff have been let go by the administration and the cost-cutting has been everywhere, I’m sure. Certainly it just falls back on the physician eventually.
KUZEL: [Is there] anybody else who takes calls that specifically deal with toxicity? I always marvel that at [Memorial Sloan Kettering Cancer Center in New York, New York], they had a [dedicated] adverse event, immunotherapy inpatient service….Does anybody have something [similar to] that at their institution or organizations? Is there somebody in the practice who specializes in that? Or it’s just you all take care of your patients and learn how to manage it?
SHULMAN: Managing the toxicities is straightforward with immunotherapy. I think with the TKIs, I’ve always had a low threshold [to] just stop and hold, let it resolve, and then restart at a lower dose. However, I think sometimes if you get behind and if they develop bad hand-foot syndrome it seems to never go away.
REFERENCES
1. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
2. Lenvima. Prescribing information. Eisai Inc; 2021. Accessed February 23, 2022. https://www.lenvima.com/-/media/Project/EISAI/Lenvima/PDF/prescribing-information.pdf?v=2020-12-22
3. Keytruda. Prescribing information. Merck & Co Inc; 2022. Accessed February 23, 2022. https://www.merck.com/product/usa/pi_circulars/k/keytruda/ keytruda_pi.pdf
4. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen














