Roundtable Discussion: Madan and Participants Debate Treatment Options for a Patient With Multiple Myeloma
During a Targeted Oncology case-based roundtable event, Sumit Madan, MD, discussed the case of a 55-year-old patient with multiple myeloma who was refractory to multiple lines of therapy.

Sumit Madan, MD (Moderator)
Hematologist/Oncologist
Banner MD Anderson Cancer Center
Banner Gateway Medical Center
Gilbert, AZ

AMBIKA: I don’t understand the rationale for going with carfilzomib [Kyprolis] in this setting. Which data are you going with in standard risk?
MADAN: The data essentially come from the FORTE trial [NCT02203643].1 The FORTE trial was 3 arms. One of the arms received KRd [carfilzomib, lenalidomide, and dexamethasone] with transplant followed by consolidation.
The second arm received only KRd for 12 cycles. The third arm received only KCd [carfilzomib, cyclophosphamide, and dexamethasone]. KCd was inferior, so we can leave that out. Within those 2 arms, KRd with transplant followed by KRd, had deep remission and deep response rates. That’s one.
We don’t have a trial comparing KRd vs VRd in patients undergoing stem cell transplant. But if you look, we have a trial showing [a] doubling of progression-free survival [PFS] advantage when Kd [carfilzomib and dexamethasone] was compared with Vd [bortezomib and dexamethasone] head-to-head in the relapsed/ refractory myeloma setting.2 The PFS with Kd was 18.7 months vs 9.4 months [with Vd]. Again, this is the relapse setting. It’s just based on my experience and looking at all the data I can get.
AMBIKA: For the quadruplet regimen right now, it’s in the high-risk subgroup, at least in the National Comprehensive Cancer Network [NCCN] guidelines, so we can appeal it.3 Otherwise, it’s hard to get insurance authorization for standard-risk patients right now.
MADAN: What is also very important is—it doesn’t happen too often, but every now and then—despite everything you do, one of your patients is going to have very bad neuropathy from the bortezomib. Recently, I had a patient that moved from California. He’s in a wheelchair from all the bortezomib he has received. I’m not saying I don’t do VRd, but I try to avoid it. [However], I don’t do CyBorD [cyclophosphamide, bortezomib, and dexamethasone] because now we have enough data—unless the patient is in renal failure, [then] yes. But cyclophosphamide is an inferior partner to bortezomib.
AMBIKA: We are using 4 drugs in the high-risk category, [such as] double-hit lymphoma or the fluorescence in situ hybridization panel showing the high-risk category. Because it’s in the NCCN guidelines, it’s easy to get it authorized. Otherwise, it’s a struggle to get it authorized for standard risk.
MADAN: Is anybody else doing 4-drug inductions for high risk or standard risk?
GROVER: I have used 4 drugs as daratumumab [Darzalex] and CyBorD for myeloma associated with some amyloidosis.
MADAN: I have not used too many 4-drug regimens. I have used it, but not up front. If I do see a plateau of the response, I [will] add it. But at this point, this is something that is in flux, and practices probably will change, as well, sometime. It’s not approved, but it’s something that probably looks like it’s going to get approved soon.

MADAN: In terms of maintenance therapy, is everybody doing maintenance therapy post stem cell transplant? If yes, how many drugs? One or 2? How are we doing maintenance, and how long are we doing it for?
KAHN: Yes, I do maintenance after transplants. It’s usually lenalidomide, similar to what you have [in this] case.
MUKHERJEE: I use lenalidomide for high-risk disease [posttransplant]. I also include daratumumab if I’ve used it in the first-line setting.
MADAN: That’s a good point you bring, because there is a clinical trial being [planned]—it’s daratumumab/lenalidomide vs single-agent lenalidomide maintenance. That’ll be interesting.
More recently, they had data on maintenance therapy post second autologous stem cell transplant [showing] movement in the PFS. Other data came from the CASSIOPEIA trial [NCT02541383].4 Patients who got daratumumab during induction don’t seem to benefit from receiving daratumumab as maintenance. We don’t have enough follow-up at this point, but that was surprising to see those patients aren’t getting a benefit. You can get benefit from either getting daratumumab in induction or getting daratumumab during maintenance therapy, [but not both]. So that was interesting.
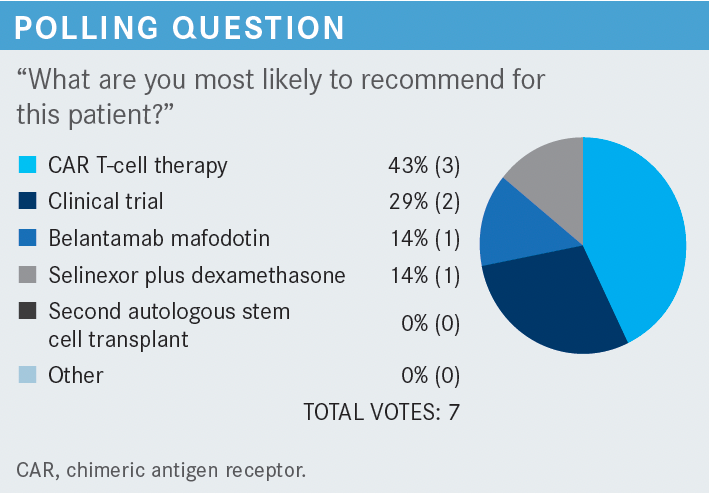
MADAN: We have 29% [favoring] a clinical trial, which is what I would’ve gone with, and 43% going in with CAR [chimeric antigen receptor] T-cell therapy. That’s interesting. Belantamab mafodotin [Blenrep] had 14% and selinexor [Xpovio] plus dexamethasone, 14%. Who would have chosen selinexor for this patient?
YOO: I picked selinexor. These are good choices. Clinical trial would’ve been my answer [in practice], but if I had to pick a standard of care, selinexor is toxic but it’s a standard option. The patient needs to receive 4 agents [for belantamab], if I’m not mistaken. Technically, I don’t think CAR T is readily available for a standard-of-care setting. That’s why I picked a standard option.
MADAN: Selinexor plus dexamethasone is an interesting choice. When this combination was first approved, this was based on a small phase 2 trial, with a response rate of [approximately] 26%, so the drug is not bad.5 The problem with using selinexor plus dexamethasone is that it’s approved for twice a week, and twice-a-week selinexor therapy is not easy for somebody with an ECOG performance status of 2. You have adverse events of nausea, vomiting, hyponatremia, thrombocytopenia, fatigue, etc.
What is really impressive is what they’ve shown with STOMP [NCT02343042].6 STOMP is selinexor once weekly, which is much better tolerated. It’s in combination with other therapies, such as carfilzomib and/or pomalidomide [Pomalyst]. Another trial is the BOSTON trial [NCT03110562], where selinexor once a week has been combined with bortezomib.7 [However], based on this patient having del(17p), selinexor could be an option, because they have shown patients with del(17p) having better outcomes. Selinexor plus dexamethasone may not be the best option, but it’s still an option. You may want to combine it with something else.
NATH: This patient had a first relapse, was treated with DVd, and had a response. That is one situation when the [Center for] International Blood and Marrow Transplant Research retrospective analysis of second transplant [could be a possibility]. Do you have thoughts on a second transplant?
MADAN: I would look for at least 3 to 4 years PFS with the first stem cell transplant, which this patient has had. So this patient is right at the cusp. At least 3 to 4 years, if you compare with the data that are coming from daratumumab-based therapies in first relapse, and if you look at POLLUX [NCT02076009].8 In POLLUX, the median PFS at first relapse is close to 4 years. That’s what I’m looking at. If you’re getting about 3 to 4 years from the first transplant, we’re probably going to end up getting 1 to 2 years from the second transplant. It’s never going to be the same number that you’re going to get from the first transplant. That is what you need to weigh against what you’re going to do. At least 3 to 4 years with the first transplant. A second transplant would be an option.
NATH: We can’t—or don’t want—to compare apples to oranges. When we say second transplant, I’m not saying just standard transplant, leaving, and letting the patient go. In the current age, once a patient with myeloma has progressed, you will need to have a treatment all the way through. So, [the] first time around, he had a VRd and autologous stem cell transplant, followed by lenalidomide maintenance. The second time [around], you want to do a transplant and you must up the ante of the posttransplant. Rather than maintenance, you will need a consolidation. I would argue that the daratumumab data should be following the other transplant to give him the best benefit.
MADAN: That’s fair because after the second transplant, I’ve always used maintenance with daratumumab—never with just an IMiD [immunomodulatory drug] or PI [proteasome inhibitor].

MADAN: What are [some] things you look for when you are deciding on a therapy for this patient?
MUKHERJEE: I definitely look at risk factors. If he has cardiac risk factors, I would go for carfilzomib, [as well as] prior mechanism of action. I would like to not repeat the same. If the duration of relapse is short and he’s high risk, I would discuss a second transplant or CAR T-cell therapy.
The problem with CAR T-cell therapy is availability of those services in our town. I think a clinical trial at a nearby center is much more readily available than CAR T. The process of CAR T is still tedious in our state.
MADAN: Where do you refer for CAR T-cell therapy?
MUKHERJEE: Our CAR T-cell therapy is in Tennessee. It [usually] takes about 2 months for processing, screening, and time to get the patients there. So patients who have relapsed usually don’t have that much time to waste. That’s what I found with another patient who had T-cell acute leukemia. By the time I got the process—not initiated, but ongoing—he relapsed, and we had to bring him in for aggressive chemotherapy. So this is a difficulty we face at this point.
MADAN: You’re right. Has anybody else had any similar issues with referring patients to CAR T-cell therapy?
BOMGAARS: Yes, I’ve had the same issue. Out of the 5 patients I referred, there hasn’t been anybody with myeloma who was fortunate enough to get CAR T. The only patient I have who got CAR T had diffuse large B-cell lymphoma.
NATH: The commercially available CAR T has a limited production capacity, so even locally, we have 2 centers that have it. It takes quite a long time. The other thing I want to [mention is] we have 2 CAR T therapies on clinical trial, and 1 is an off-the-shelf CAR T for patients who have had 2 prior therapies—a clinical trial certainly is available locally for [some patients].
MADAN: Absolutely. The only other thing I would add is that, regarding the clinical trials, I’ve noticed that nationally, whenever they have a slot open up, they want someone enrolled within 3 or 4 days. You either enroll or you lose it. It’s difficult to enroll in a clinical trial, even the [trial for] idecabtagene vicleucel [Abecma], because of the [production] shortage throughout the world, and it’s not activated in some locations. The CAR T is promised a lot, but it’s hard to deliver it.

MADAN: Renal impairment is very common. At least half of patients with myeloma are going to have some degree of renal impairment. How do you treat those patients? Are there any drugs you would avoid specifically? Are there any drugs you would prefer in patients with renal failure?
SHARMA: Medications like lenalidomide become a little problematic. You must look at what does not affect the immune [system as a part of] their evaluation—as far as the renal failure, too—and look for whether they have other issues or comorbidities [such as] diabetes and other things.
MADAN: We are very fortunate that most of our drugs are safe, at least in mild to moderate renal impairment. If you look at what is [available, it is] completely safe. IMiDs, except for lenalidomide, and pomalidomide are safe. Monoclonal antibodies are one of the safest drugs in renal failure, both isatuximab [Sarclisa] and daratumumab—even elotuzumab [Empliciti]—and belantamab and selinexor are also safe in these patients.
In these patients, who have had 1 to 3 prior lines of therapy, we already know it’s a very competitive space. All these drugs and their combinations, such as daratumumab, isatuximab, pomalidomide, lenalidomide-based combinations, elotuzumab, selinexor—all these are in the NCCN guidelines as category 1.3 When those patients relapse, we’re looking at patients who have progressed on an anti-CD38, a PI, and an IMiD. This opens up a whole new space.
The first drug that was approved was belantamab. That was approved in 2020. Then came the idecabtagene vicleucel. The other combination—selinexor and dexamethasone—can be used in patients who have progressed on 2 PIs, 2 IMiDs, and anti-CD38. However, I don’t think there’s too many people who would use just selinexor and dexamethasone.

AMBIKA: Doing the Risk Evaluation and Mitigation Strategies [REMS] criteria is a pain.9 We tried giving it, and we kind of gave up.
For community practices, it’s so hard to have the staffing and enough ophthalmologists on board for weekly eye testing. REMS is the main issue. Otherwise, it’s a good medicine, at least in the current setting.
ARSLAN: The PFS [with belantamab] is 2.8 months, so why use this medication over elotuzumab or anything else?10,11 That part is not very clear. The response rates are not high. They’re approximately 30%. But if you combine it with bortezomib, there may be a better response rate. But if you use it as a single agent, the PFS is 3 months. [It doesn’t] provide much benefit in that scenario.
MADAN: I would like to clarify the eye examination is to be done once every 3 weeks, so we do it prior to starting therapy, then before each dose.9 Now the median PFS is approximately 3 months, but the median OS and the depth of response was still impressive. Remember, this was a highly refractory patient population, [with a] median of 7 prior lines of therapy.10,11
BOMGAARS: I had a patient use belantamab, and it was interesting. I thought he responded well. His light chain [and creatinine] got better. He was progressing rapidly, and I thought he was going to be on dialysis next week, then his anemia worsened to the point that he needed a transfusion. But everything got better after just 1 dose of belantamab. After the second dose, the hemoglobin went up to 9 g/dL, and the creatinine went down to 2 g. What was odd was the M protein suddenly surged up it went to 2.9 g/dL from 2 g/dL. Initially, it was coming down from 2.5 g/dL to 2 g/dL, then suddenly it bounced right back up. I was concerned, and I talked to Mayo Clinic and asked them whether this was usual or whether this was a sign of progression? The [physician] told me it was progression, so that’s why he’s on selinexor now. But it was odd because everything was better.
MADAN: How many doses were you able to give him? Did he have any ocular toxicity?
BOMGAARS: Two. And no, he tolerated it. He was just really tired. Otherwise, he tolerated it well.
MADAN: That’s good. I’ve had patients on belantamab. I have 1 patient right now who’s been on this drug since April, and he’s done phenomenally well. I have another patient who was doing well, got ocular toxicity, and within a span of approximately 6 or 7 months, got belantamab maybe 3 times, and still responded. That’s exactly the data we see from this drug. [Although] the patient may not be able to get the drug, the drug is still working, so there’s still a deepening of the response in the responders. That’s important to understand. Patients are going to have ocular toxicity. You will have to hold the drug. [Unfortunately], that’s one of the things with this drug. But once the patient responds, the depth of response and the duration of response, at least on the trial, was pretty impressive— close to a year.
BOMGAARS: Do you know whether it [affects] the M protein, such as elotuzumab or daratumumab, at all?
MADAN: It is just like an IgG, but not at that level. When we detect daratumumab, [we see] very small levels in patients who are in complete remission, and suddenly we start this drug and see a small M spike or immunofixation positive, but not to the point where the M protein is going to go up from 2.2 g/dL to 2.9 g/dL. That would be very unusual.
Does anybody want to share their perception of this agent? We know it has ocular toxicity, but what else?
GROVER: I have not used this drug yet, so I don’t have any experience. But my main worry was—there’s 1 patient I was discussing, then she read about the ocular toxicity and was reluctant to consider. So that has been an issue with some of the patients, as well. But no, I don’t have any other concern at this time.
AMBIKA: Is there any other toxicity from payload? No interstitial lung disease or anything with this?
MADAN: No. If you look at the adverse event profile, it’s just the eye toxicity and thrombocytopenia. Not much of anything else.
REFERENCES
1. Gay F, Musto P, Rota-Scalabrini D, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22(12):1705-1720. doi:10.1016/ S1470-2045(21)00535-0
2. Chng WJ, Goldschmidt H, Dimopoulos MA, et al. Carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia. 2017;31(6):1368-1374. doi:10.1038/leu.2016.390
3. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 4.2022. Accessed December 14, 2021. https://bit.ly/3JZWq3p
4. Moreau P, Sonneveld P. Daratumumab (DARA) maintenance or observation (OBS) after treatment with bortezomib, thalidomide and dexamethasone (VTd) with or without DARA and autologous stem cell transplant (ASCT) in patients (pts) with newly diagnosed multiple myeloma (NDMM): CASSIOPEIA Part 2. J Clin Oncol. 2021;39(suppl 15):8004. doi:10.1200/JCO.2021.39.15_suppl.8004
5. Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. doi:10.1056/NEJMoa1903455
6. Chen CI, Bahlis N, Gasparetto C, et al. Selinexor, pomalidomide, and dexamethasone (SPd) in patients with relapsed or refractory multiple myeloma. Blood. 2019;134 (suppl 1):141. doi:10.1182/blood-2019-122907
7. Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563-1573. doi:10.1016/S0140-6736(20)32292-3
8. Bahlis NJ, Dimopoulos MA, White DJ, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34(7):1875-1884. doi:10.1038/s41375-020-0711-6
9. Risk Evaluation and Mitigation Strategy (REMS) document: Blenrep (belantamab mafodotin) REMS program. FDA. Accessed January 25, 2022. https://bit.ly/3GWBpFD
10. Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207-221. doi:10.1016/S1470-2045(19)30788-0
11. Lonial S, Lee HC, Badros A, et al. Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer. 2021;127(22):4198-4212. doi:10.1002/cncr.33809















