Roundtable Discussion: Isaacs Discusses Second- and Third-Line Therapy for Patients with TNBC
During a Targeted Oncology case-based roundtable event, Claudine Isaacs, MD, discussed treatment options for a patient with triple-negative breast cancer whose condition worsened after first-line gemcitabine plus carboplatin.

Claudine Isaacs, MD
Professor of Medicine and Oncology
Associate Director, Clinical Research
Leader, Clinical Breast Cancer Program
Lombardi Comprehensive Cancer Center
Georgetown University


GORBATY: I would strongly consider sacituzumab [Trodelvy], because [the patient is failing treatment] in less than 12 months from active chemotherapy.
ISAACS: What do others feel?
KATRAGADDA: I agree with sacituzumab in this setting, as she had early progression within less than 12 months, and she qualifies for its labeled indication.
SMITH: [In my opinion], unfortunately, this patient is not curable. I think sacituzumab is still [the correct choice to me] and she has got a good performance status. Capecitabine is also a great drug choice.
HAGAN: I love the idea of capecitabine, but I’m also thinking this patient might do potentially better with eribulin [Halaven], because that in a clinical trial, I thought, performed better than capecitabine.1 I could potentially use capecitabine at a time when the patient is a little bit more beat up and looking for something that was a little easier to go with, but I totally love the idea of capecitabine. I’d have to have a good conversation with the patient that that might not, survival-wise, be the best bet, potentially.
GORBATY: What do you think the response rate to capecitabine in this setting would be?
ISAACS: This is a patient who obviously has a very poor prognosis, and who relapses so quickly after dose-dense [chemotherapy treatment]. I think it is unlikely that she would have a response, but the issue with what we do is that we don’t have good markers of who is going to respond to which therapy, and I think we all recognize that that’s where we’re trying to get to. However, I think she probably has a small chance of responding to capecitabine, but I understand the rationale that people are using for this, and sacituzumab is currently approved after 2 prior lines of chemotherapy, including 1 in the adjuvant setting.2
I think we’ve all had experiences where we might have requested that drug and been able to get it. I think that there were some good points brought up about eribulin, but the question now is how we would sequence things, and I think one of the things that I always think of with our metastatic patients is we need to think about sequencing, but we’re typically not. But I think people had a good rationale for the various approaches. I think that personally, I would have tried to put her on a clinical trial, because obviously, she doesn’t have very chemotherapy-sensitive disease. We recognize that we don’t have trials available for these people, so we don’t always have that as an option, but in terms of other things, I probably would have leaned more toward sacituzumab or eribulin, but I think capecitabine is a very reasonable choice in her as well.
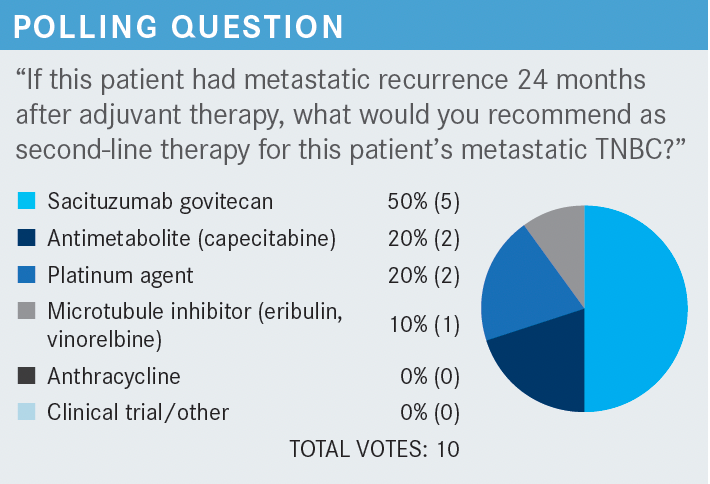
ISAACS: For those of you who chose combination chemotherapy, what were you thinking of in this patient? And let’s turn it back to [when] after 8 months, she progressed, as opposed to now. What combinations are people thinking about?
SMITH: Well, I didn’t choose that, but the immediate thing is to try to grab for a platinum therapy, right? So carboplatin [in my opinion].
ISAACS: We didn’t have a chance to talk about that there, but the concept, you’re using carboplatin for TNBC.
SMITH: Or that in fact that she did have paclitaxel, and try to use nanoparticle paclitaxel.
ISAACS: What would your front-line treatment have been between 8 months and 24 months for relapse?
SUDARSAN: I would probably be keener to use chemotherapy here on a patient that had that 2-year gap between her adjuvant therapy and a relapse. I would probably go toward something like eribulin.
WENG: It depends on [the patient’s] goals, and it sounds like she’s active, good performance status, young, and probably, I presume, wants to be very active, so oftentimes, for those patients, I go with oral capecitabine to give them the most latitude for their activities, and to be able to titrate the tolerability. I do restage relatively soon after starting, just to make sure I’m on the right track, because my other go-to for single agent is eribulin. I’ve had a good experience with that, but it does tie patients up, and does have greater intolerability compared with capecitabine.
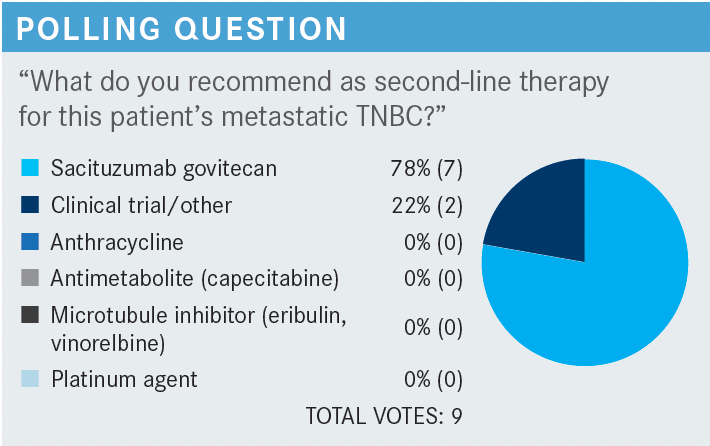
ISAACS: So let’s think about the patient with rapid recurrence, and the question about why you made these decisions and at this point, [have chosen sacituzumab govitecan]. What is it that’s guiding this recommendation for you?
BHANDARI: [The patient in this case] has progressed quite oddly after the initial therapy of 8 months. She has, I would say, an overall poor prognosis. If the clinical trial is not available, I think I will probably go with a combination of carboplatin/gemcitabine, because she had not received carboplatin/platinum and gemcitabine, so that would be one option. That could be considered a single-agent treatment, but I will probably go with the doublet to start with and see how she responds, and sacituzumab, I will probably do later.
ISAACS: This is a patient who we chose to give that combination chemotherapy to on her first line as she progressed after 8 months, and now you’re choosing the second-line therapy, and for this question, most of you chose sacituzumab, and about one-fifth of you chose clinical trial or other.
BHANDARI: I picked sacituzumab because my thinking was, she is going to have adverse effects [AEs] from other microtubule inhibitors. She may not be able to go through another agent that can worsen neuropathy, so [I would still pick] sacituzumab, I thought I will have a better chance of getting a better response rate, and still maintaining her quality of life.
KALIL: For example, we don’t know which treatment is the best option, so we try to figure out which would cause less AEs, and not compromise the patient’s quality of life. Other than that, whatever you decide, we have been talking, is just absolutely a personal decision, not based on any clinical trials. That’s my feeling because we don’t know which treatment is the best. There’s no right and wrong answer here, so that’s why I choose sacituzumab. But really, if you ask me, is this the best option for the patient? Well, if you consider AEs it is a good option.…Again, the patient is not curable, so we need to go to sequential therapy and minimize complications from the chemotherapy, but if I knew that one is better than the other, then which is for the best, but we don’t know.
ISAACS: Does anybody else have thoughts or comments about that, or how they made their choice here?
HAGAN: I picked sacituzumab in this situation because my memory of the clinical trial was that sacituzumab was being compared with physician’s choice of single-agent chemotherapy and won out on both progression-free survival [PFS] and overall survival [OS], so we do have a little bit of a hint as to, at least for single-agent chemotherapy, sacituzumab beat out what the physicians chose. I mean, that’s kind of a real-world study in some ways.
Use your best option if you don’t choose sacituzumab, and there was an OS advantage of something like 12 months vs about 7 months. I don’t know how many people in the physician’s choice arm then might have been able to go on to get sacituzumab second line, further line, so it was a question of early vs late, but at least we do have a hint, if you didn’t have that option, that sacituzumab was going to win out. So again, what I would have done otherwise, which again, probably would have been eribulin.
ISAACS: I know there has just been a rush of different drugs out there, and lots of different trials, but there is the ASCENT trial [NCT02574455] that did look at that and found what you said, and my guess is that most patients didn’t go on to get sacituzumab, because it wasn’t available.3 These patients tend to progress quite quickly, so it’s fairly unlikely that they did, although some might have gotten it subsequently after its approval.
Do people want to talk about what they think is the ideal sequence of drugs for a patient who [we have stratified based] on their PD-L1 status? We have one sort of algorithm that we go down if they are PD-L1 positive, and a different one if they are PD-L1 negative. So for PD-L1–negative [patients in the first line] it sounded like people are using a variety of different choices, including eribulin, capecitabine, some carboplatin, and then in the second line, it looked like, from the poll, that many people were choosing sacituzumab. However, for a patient who had had disease progression, her original disease-free interval was longer and there was more variability. Do people have a set algorithm [to look at their PD-L1 status]?
GAI: It’s quite individualized to me, for example, if somebody has a large tumor burden, I’d probably try the doublet like you did, carboplatin, the gemcitabine, first-time palliative chemotherapy. After that, I’m not sure the doublet is going to offer any benefits. I will try the single agent, but if the patient has a quick progressive disease, I will probably avoid the capecitabine or the pill form. I’d try the IV drug, and I’d try to switch to sort of a fundamentally different mechanism of action, like sacituzumab, rather than something else. That’s my sort of philosophy.
ISAACS: Does anyone feel differently? Is there any place where you would all put 1 drug? Like, there’s variability, but is your second-line choice always this, or is there anything, or most often, there’s never always in medicine, and I always tell the medical students and residents that there’s no always and nevers in medicine. However, your general go-to in the first line or second line or third line, but variability in some of the other lines, anything that people are thinking about that, and what drives that choice, if that’s what they’re doing?
WENG: One of the items is, as somebody mentioned, the disease burden and how aggressive the patient wants to be. I think that the carboplatin/gemcitabine combo is very active, and it does have a higher response rate in my experience, but also more toxicity. You can have very motivated patients who want to have the most aggressive chemotherapy approach, because not only do they have large disease burden, but they do feel that they want to be as aggressive as possible. So that gives you that option, but there are other patients who don’t want as much toxicity, so more customized chemotherapy gives them more flexibility for their lifestyle, and if they haven’t had capecitabine before, then that’s an option.
There are a lot of triple-negative patients who get capecitabine because they got neoadjuvant therapy and then had a partial response, so it’s always a matter of, I think, disease burden is a factor, too, to choosing therapy, and that’s always a consideration.

GORBATY: I was happy about it. I think the earlier you use it, it gives more patients an opportunity to benefit from it, and I think it may eventually be a first-line option, though it isn’t currently approved for that. So I was wondering, do we know what the response rate or PFS is when you use it first line vs second vs third?
ISAACS: I think maybe we’ll get some forest plots coming along that will show, I think, one of the breakdowns, so we can look at that in a second. But if I’m interpreting right, Mayer, what you were also interested in, or what was good about this, was that it was approved earlier on than the conditional approval. It allowed you to give it after just 1 line of therapy, and that was something that you found appealing. Is that right?
GORBATY: I mean, we all know that with each line of therapy, people fall off, and they never get a chance to be exposed to a drug, so the earlier you can give it, potentially the more people can benefit.
KATRAGADDA: It opens more opportunities for the patients to receive this if it is approved in the first-line metastatic setting.…I thought it was…he could use it in the first line, if you had progressed within 6 months to a year after adjuvant, that could be counted as 1 line of treatment, but as the previous colleague has mentioned, of patients from each line of treatment, so the sooner you get it the better it is, given that it has beaten out the standard of care or physician’s choice treatments.
REFERENCES
1. Pivot X, Im SA, Guo M, Marmé F. Subgroup analysis of patients with HER2- negative metastatic breast cancer in the second-line setting from a phase 3, open-label, randomized study of eribulin mesilate versus capecitabine. Breast Cancer. 2018;25(3):370-374. doi:10.1007/s12282-017-0826-4
2. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA. April 8, 2021. Accessed February 17, 2022. https:// bit.ly/3sJiloq
3. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021 Apr 22;384(16):1529- 1541. doi:10.1056/NEJMoa2028485


















