Pal Evaluates Frontline TKI/IO Combinations for Favorable-Risk mRCC
During a Targeted Oncology™ Case-Based Roundtable™ event, Sumanta K. Pal, MD, discussed the updated results of frontline trials of patients with metastatic renal cell carcinoma.
Sumanta Kumar Pal, MD
Professor, Department of Medical Oncology and Therapeutics Research
Codirector, Kidney Cancer Program
City of Hope
Duarte, CA

CASE
- A 61-year-old man presented with a history of metastatic renal cell carcinoma (RCC). The patient, who had an active lifestyle, was post left nephrectomy and adrenalectomy. There were clear cell RCC (ccRCC)/metastases in his adrenal glands.
- Four years later, the patient had a recurrence of lung nodules, and a biopsy showed they were consistent with ccRCC. In retrospect, the cancer had been present on scans for at least 2 years prior to biopsy.
- The patient was observed based on the low-volume and indolence of the disease and the patient’s preference.
- Eighteen months later, reexamination showed continued indolent growth on scans, increased total tumor burden, and a new paratracheal lymph node (2.0 × 1.5 cm).
- ECOG performance status: 0
Targeted OncologyTM: What did the results of the CheckMate 9ER trial (NCT03141177) of cabozantinib (Cabometyx) plus nivolumab (Opdivo) demonstrate?
PAL: The CheckMate 9ER trial [shows] the evidence [in support of using cabozantinib/nivolumab] in the frontline setting. This study took patients with previously untreated disease with a clear cell component; patients could be in any risk group. The dose of cabozantinib, 40 mg, was lower than the 60 mg that we are used to using in the second-line setting.1 The comparator arm, as across all the studies, was sunitinib [Sutent], 50 mg daily, 4 weeks on, 2 weeks off.1-3 The primary end point in this study was progression-free survival [PFS], with secondary end points of overall survival [OS], objective response rate [ORR], and safety.1
There was a doubling of the PFS [8.3 months vs 16.6 months for the comparator vs experimental arms, respectively; HR, 0.56; 95% CI, 0.46-0.68].4 This HR was a bit better than what we saw with axitinib [Inlyta] plus pembrolizumab [Keytruda].5 The HR was not quite as good as what we saw with lenvatinib [Lenvima] plus pembrolizumab,6 but there are reasons for that, and I will highlight those.
The OS data showed that there was a clear advantage with cabozantinib/nivolumab over sunitinib [median OS, 37.7 months vs 34.3 months, respectively; HR, 0.70; 95% CI, 0.55-0.90].4 The HRs for all these studies coalesce around [0.70]. So no matter what the difference is in PFS among cabozantinib/nivolumab, lenvatinib/pembrolizumab, and axitinib/pembrolizumab, and so on, I think we are having the same impact on OS with these regimens.4-6 That’s a key point to keep in mind.
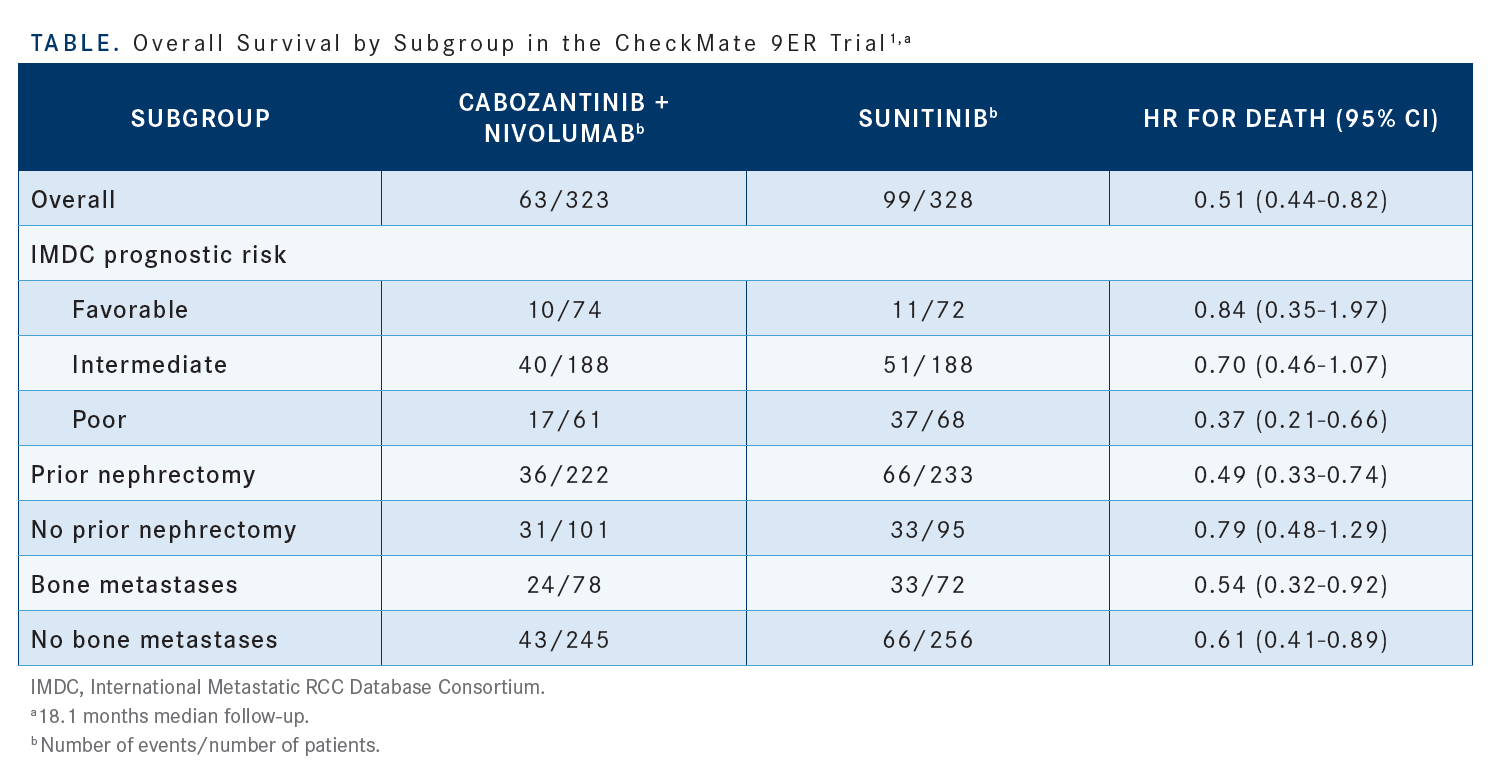
The [subgroup analysis] tells a very important story here [Table1]. One of the things to keep in mind is that we looked at this regimen in a fairly large proportion of patients who had no prior nephrectomy. [Regardless of whether] a patient did…or didn’t have a previous nephrectomy, there was still a benefit with this regimen [HR, 0.49 vs 0.79, respectively].1 I think that the fact that we have so many patients without prior nephrectomy in this cohort, about 30% of the study population, makes it more challenging for us to ascertain a response. This might have been the group of patients with the poorest prognosis among all the patients in the frontline phase 3 clinical trials. There were also a considerable number of patients with bony metastases. That’s one of the things that we’ve always heralded about cabozantinib: It blocks signaling through MET, and MET seems to be overexpressed in bony metastases. There seems to be similar benefit in patients with [vs without] bony metastases [HR, 0.54 vs 0.61, respectively].1
The ORRs all coalesce across these studies between 50% and 70%. It’s 70% with lenvatinib/pembrolizumab, but, and this is an important point, in the cabozantinib/nivolumab clinical trial 20% of patients had favorable-risk disease, and in the lenvatinib/pembrolizumab study 30% of patients had favorable-risk disease.1,3,6 Presumably, it’s easier to get a response in a favorable-risk population, so that might have been a key driver. I think the ORR [in the CheckMate 9ER trial] was respectable [55.7%; 95% CI, 50.1%-61.2%].4 We always used to think about nivolumab plus ipilimumab [Yervoy] and its ability to induce complete responses [CRs]. The CR rate here was [12.4%] and looks very similar to that of nivolumab/ipilimumab and a little better than that of axitinib/pembrolizumab.4,5,7
Some of the more recent analyses have looked across different subgroups [defined with respect to site of metastasis:] liver, bone, or lung. There is benefit with cabozantinib/nivolumab that is [somewhat] consistent across subgroups.7 For a patient with aggressive bony metastases, I have a predilection toward cabozantinib based on evidence such as this.
What did this study demonstrate about the safety of the cabozantinib/nivolumab combination?
The thing to keep in mind with tyrosine kinase inhibitor plus immune-oncology [TKI/IO] combinations, beyond the expected additive toxicities, fatigue, and so forth, [is there will] be a much higher incidence of severe diarrhea. And the other things that stand out with these TKI/ IO combinations are increases in aspartate aminotransferase [AST] and alanine aminotransferase [ALT].4 Those are much more pronounced with the TKI/IO combinations than with monotherapy with either of those agents. One thing that speaks to the tolerability of this regimen is that [only] 7.5% of patients had discontinuation of both cabozantinib and nivolumab in this context,4 which I think is quite compelling.
Are the elevations of AST and ALT attributable to autoimmune reactions?
My belief is that it is a synergistic toxicity. If you take either one of those 2 therapies, there is a certain incidence of hepatotoxicity, but it is more pronounced when you combine the agents. And that is true for axitinib/pembrolizumab and for lenvatinib/pembrolizumab. It dissuades [me from] the practice of using agents such as sunitinib and pazopanib [Votrient] after IO-based therapies. I have seen that in some settings. Physicians often give pazopanib to patients after axitinib/ pembrolizumab or after cabozantinib/nivolumab. What we showed in a couple of studies is that if you look at the combination of sunitinib plus nivolumab or the combination of pazopanib plus nivolumab, there are high rates of hepatotoxicity with those drugs.8 Cabozantinib, lenvatinib, and axitinib seem to be much more combinable. So if you have a patient who has just progressed on IO therapy, try to stay away from sunitinib or pazopanib because those agents are probably going to prompt liver function test results to [go up]. There is probably still some lingering IO [activity] there.
Do any of these agents cross the blood-brain barrier?
There was only 1 formal study [of this], which was published by [investigators from] the Dana-Farber Cancer Institute in JAMA Oncology in [December 2021]; that study looked at cabozantinib in the context of brain metastases.9 The same may be true for axitinib and for lenvatinib. We just don’t have the evidence for it. But it does appear that patients who are receiving cabozantinib have intracranial reductions in tumor burden. Cabozantinib seems to have that unique property. There are 3 places that we usually think of [as having] more pronounced MET expression. One is in the context of bony metastases, another is possibly in liver metastases, and a third is potentially in brain metastases. I do think there’s some good evidence for cabozantinib to work in those contexts.
Does either nivolumab or pembrolizumab have efficacy in the brain?
There was a study [ABC; NCT02374242] looking at nivolumab in the context of brain metastases, and also [another study; (CheckMate 204; NCT02320058)] looking at nivolumab/ipilimumab in the context of brain metastases, and many have seen [these] melanoma data.10,11 One of my colleagues, Kim Margolin, [MD,] was the senior author on [the CheckMate 204 report]. And she would always tell us about how nivolumab/ ipilimumab has great activity in metastatic disease. But I think that’s a melanoma-specific phenomenon. In renal cell carcinoma, the activity of nivolumab/ipilimumab in brain metastases seems to be very modest. We don’t get a lot of activity from IO therapy alone in that setting.
What did the KEYNOTE-426 (NCT02853331) trial reveal about the efficacy of the axitinib/ pembrolizumab combination in the treatment of newly diagnosed or recurrent stage IV ccRCC?
I think [many physicians] are familiar with axitinib/pembrolizumab and use it frequently. The KEYNOTE-426 study started with axitinib at 5 mg oral twice daily with dose titration, just like you can with axitinib in the second-line setting. [The axitinib dose could be increased] up to 7 mg, [then] up to 10 mg, or you could dose reduce, as you would in a standard fashion, down to 3 mg or to 2 mg. This was paired with pembrolizumab, and this combination was compared with sunitinib.2
One of the things I will point out is that the HR was maybe a little less impressive than those of other trials. For OS, the HR was 0.73 [95% CI, 0.60-0.88; P < .001], which is on par. But the HR for PFS was on the same order [HR, 0.68; 95% CI, 0.58-0.80; P < .0001]. The difference in median PFS seems to be more modest [11.1 months vs 15.7 months for the comparator vs combination arms, respectively].5 The HR was not quite as good as what we saw with cabozantinib/nivolumab.4
The OS was assessed by subgroup. Nothing stands out to me as being markedly different. It looked as though favorable-risk patients didn’t benefit quite as much from axitinib/pembrolizumab as did intermediate- or poor-risk patients.8 But I’d caution against overinterpreting this; these are very small subsets here.
The ORRs seemed a little higher than what we saw with cabozantinib/nivolumab, approximately 60% vs 55%.4,5 But keep in mind that this study had a larger proportion of patients with favorable-risk disease. The CR rate was respectable in the KEYNOTE-426 trial at 10.0%. I think that it’s important to point out that primary progressive disease was lower with axitinib/pembrolizumab, but lower still with cabozantinib/nivolumab and with lenvatinib/pembrolizumab.4-6
What did this study reveal about the safety of the axitinib/pembrolizumab combination?
Regarding tolerability [with respect to discontinuation], I would say that if you look at axitinib and pembrolizumab independently, they were [each] maybe a little inferior to cabozantinib/nivolumab in combination, but the cumulative rate of discontinuation of both axitinib and pembrolizumab was under 10%, which is pretty favorable. It looks to me as though the AST/ALT changes were [similar to] what we saw with cabozantinib/nivolumab, and the rates of diarrhea were quite similar.4,5 So the toxicity profiles were not markedly different.
The CLEAR study (NCT02811861) investigated the combination of lenvatinib plus pembrolizumab. What did the results reveal about this regimen?
This is probably the most [challenging] trial to interpret out of the group. When you look at the axitinib/pembrolizumab trial, you see that they were using the same dose of axitinib as we would use in the second-line setting.2 When you look at the CheckMate 9ER trial with cabozantinib/nivolumab, they were using a lower dose of cabozantinib than you would in the second-line setting,1 40 mg instead of 60 mg. What’s tricky about the CLEAR trial is that they were using a higher dose of lenvatinib than we would use in the second-line setting, going from 18 mg up to 20 mg. The primary end point was PFS.3
It is important to look at the baseline demographics for these studies when [comparing them]. The median PFS in the CLEAR trial approached 2 years [HR, 0.42; 95% CI, 0.34- 0.52],6 and it looks compelling. But this study had the highest proportion of patients with favorable-risk disease, approximately 30%, so that stacks the odds in favor of this particular trial vs the CheckMate 9ER study.1,3
Regarding the data as they pertain to certain subsets, I will point out that some of the tenets that held with the cabozantinib/nivolumab trial also hold in this population. The PFS benefit stands across the different risk categories: favorable, intermediate, and poor.3 The benefit seems to hold in the presence of bone metastases, although the numbers there are very small. It [also] holds in the context of liver metastases, and it holds in the context of having had prior nephrectomy.9
We saw a marked difference between the arms in terms of PFS, but in terms of OS, the curves come together a bit at the end [median OS, not reached for either arm].6 The other thing that I will point out is that the OS HR [0.72; 95% CI, 0.55-0.93] was virtually identical to what we saw with cabozantinib/nivolumab and with axitinib/pembrolizumab.4-6 There was not a huge amount of difference across these regimens, from that standpoint.
[An analysis was performed of] the OS in patients who completed 2 years of pembrolizumab and then continued on lenvatinib therapy [OS at 36 months, 94.5%].6 This [appeared to show] that you can still have success if you drop the pembrolizumab at the 2-year mark and just keep the patient on lenvatinib by itself. It is hard to interpret these data. First and foremost, the analysis included a small subset of individuals, 101 patients in total. And there was a lot of censoring in the [analysis]. I don’t know whether [this goes against] continuing pembrolizumab, because there is a lot of attrition and the curve does step down over time.6 But some have used this as an argument in favor of dropping pembrolizumab at the 2-year mark.
There was a [71.0%] ORR, which is the highest out of these trials.4-6 And there was a [17.2%] CR rate, but bear in mind that this study had the smallest proportion of patients who had not had nephrectomy, and it had the highest proportion of patients with favorable-risk disease.1-3,6 Many things stack the odds a bit in favor of this trial.
What did the CLEAR study and other studies show about the tolerability of these regimens?
The rate of adverse event–related discontinuations was much higher in this study than in the context of cabozantinib/ nivolumab or of axitinib/pembrolizumab, so I do think that patients are potentially paying a price from the standpoint of toxicity with this regimen.3-5 It does stand out to me that cabozantinib/nivolumab may serve us best when it comes to discontinuations related to therapy, which is an important thing to keep in mind.
Other important data, which I think we don’t cover too often in the context of these discussions, are quality-of-life data. This is a factor that does steer me toward one regimen over others. When you compare lenvatinib/pembrolizumab or axitinib/pembrolizumab with sunitinib, you do not see much of a difference or an improvement in the quality-of-life metrics for RCC. But with the cabozantinib/nivolumab data, there are some modest improvements.12-14 These data are not perfect by any means, but they give us our best sense of how these trials stack up against each other in terms of the patient experience.
REFERENCES
Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
Powles T, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): phase III KEYNOTE-426 study. J Clin Oncol. 2019;37(suppl 7):543. doi:10.1200/JCO.2019.37.7_suppl.543
Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ-specific target lesion assessments with two-year follow-up in CheckMate 9ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. J Clin Oncol. 2022; 40(suppl 6):350. doi:10.1200/ JCO.2022.40.6_suppl.350
Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(suppl 15):4500. doi:10.1200/JCO.2021.39.15_suppl.4500
Porta CG, Eto M, Motzer RJ, et al. Updated efficacy of lenvatinib (LEN) + pembrolizumab (PEMBRO) vs sunitinib (SUN) in patients (pts) with advanced renal cell carcinoma (aRCC) in the CLEAR study. Ann Oncol. 2022;33(suppl 7):S660- S680. doi:10.1016/annonc/annonc1072
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277- 1290. doi:10.1056/NEJMoa1712126
Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer. 2018;6(1):109. doi:10.1186/s40425-018-0420-0
Hirsch L, Martinez Chanza N, Farah S, et al. Clinical activity and safety of cabozantinib for brain metastases in patients with renal cell carcinoma. JAMA Oncol. 2021;7(12):1815-1823. doi:10.1001/jamaoncol.2021.4544
Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681. doi:10.1016/ S1470-2045(18)30139-6
Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722-730. doi:10.1056/NEJMoa1805453
Motzer R, Porta C, Alekseev B, et al. Health-related quality-of-life outcomes in patients with advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab or everolimus versus sunitinib (CLEAR): a randomised, phase 3 study. Lancet Oncol. 2022;23(6):768-780. doi:10.1016/S1470-2045(22)00212-1
Bedke J, Rini B, Plimack E, et al. Health-related quality-of-life (HRQoL) analysis from KEYNOTE-426: pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). Presented at: 35th Annual European Association of Urology Congress; July 17-19, 2020; virtual. Accessed February 22, 2023. https://bit.ly/3SOWn03
Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):292-303. doi:10.1016/S1470-2045(21)00693-8

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen