Clinical Commentary: Tawbi Assesses Toxicities of Therapies in Advanced Melanoma
During a live webinar, Hussein Tawbi, MD, PhD, discussed first-line therapy for patients with stage IV melanoma, particularly the impact of targeting both PD-1 and LAG3 in these patients by using nivolumab and relatlimab.

Main Melanoma Therapies
We have randomized phase 3 data for pembrolizumab [Keytruda], nivolumab, and…nivolumab plus ipilimumab [Yervoy], so I completely agree that all of these [agents are considered] category 1 treatments by the NCCN [National Comprehensive Cancer Network] because all have shown improvements over single-agent ipilimumab.1 That is kind of where the category 1 comes from when including overall survival [OS] benefit. I consider NCCN guidelines to be…very safe.… They don’t include options just because they exist.

However, [combination] pembrolizumab and low-dose ipilimumab has been tried in 1 large single-arm study called KEYNOTE-29 [NCT02089685] and [found to be]…safer than the combination of ipilimumab and nivolumab or high-dose ipilumumab.2 Yet, most of…[the] relevant data are from a second-line study that showed about a 25% response rate in the second line low dose of ipilimumab or pembrolizumab. So, personally, I don’t necessarily agree with this recommendation as a first-line regimen for low-dose ipilimumab/ pembrolizumab, but obviously this is up for discussion.
Relatlimab’s Role
Relatlimab is a novel antibody that blocks LAG3…. It’s one of those interesting receptors and is quite different than PD-1. It’s expressed on activated T cells and exhausted T cells. Initially, people were thinking that it has an association with MHC class II, the primary ligand, but more and more data are arising to show that it’s directly associated with the TCR CD3, basically signaling cascade.3 It actually modulates TCR signaling, so it makes a bigger impact in a place where there’s a lot more TCR signaling happening, and that’s probably why it works better in the first line than… in the second line.
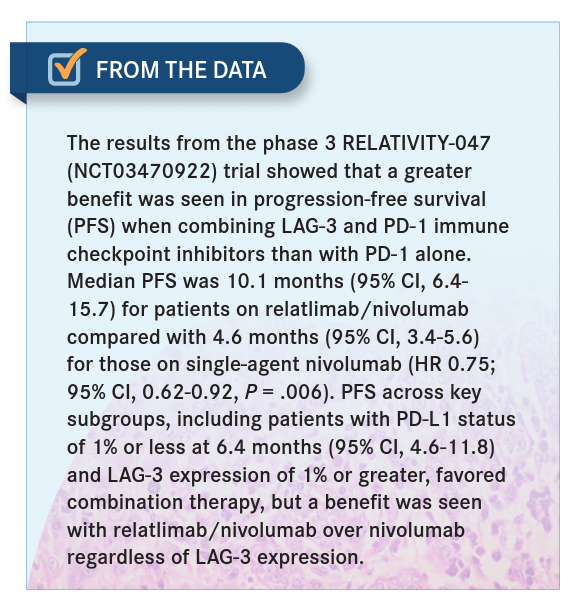
We just published in Nature [results of a] neoadjuvant study where the response rate in our neoadjuvant patients was 57%, so the earlier you use it, the more signaling happens through TCR, and the more you can modulate anti-LAG3.4 Now, the RELATIVITY-047 study [NCT03470922] was a phase 3 trial followed by the FDA approval of nivolumab plus relatlimab [for patients 12 years and older with unresectable/metastatic melanoma].5,6
Comparing Toxicities
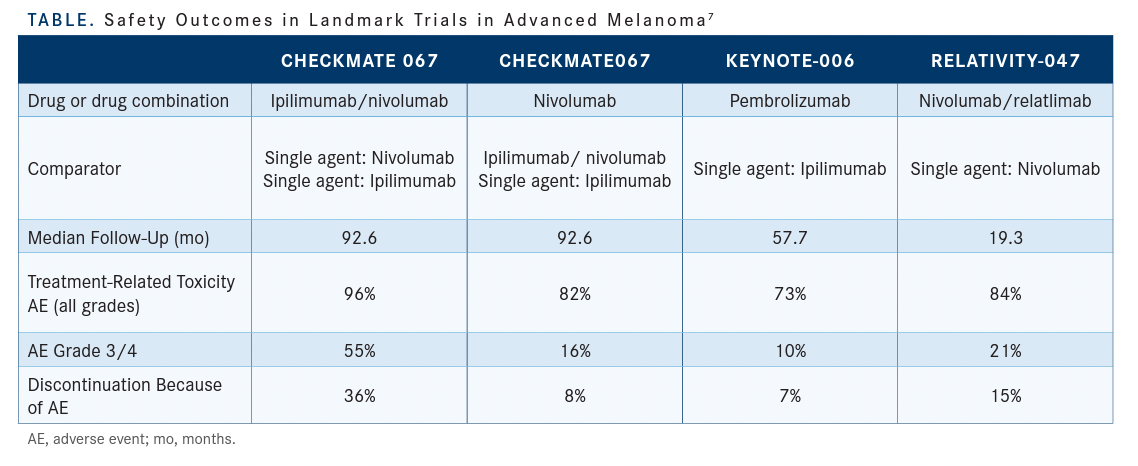
With ipilimumab/nivolumab in any setting, you get grade 3 to 4 toxicity of at least 50%, and the highest discontinuation rate because of an adverse event [AE] was 36% in the CheckMate 067 [NCT01844505] study.7 [The rate of any grade 3/4 AEs with] nivolumab and relatlimab was 21%, and this is why I do feel like it’s slightly more toxic…but the pattern of…toxicity is similar [Table7]. With [this combination] every toxicity I’ve encountered feels the same as when you encounter a singleagent toxicity. It doesn’t feel a lot more recalcitrant and it’s not a lot harder, and you get 1 toxicity per patient, just like you would get it with the single-agent PD-1 inhibitor. The other factor that I share with [patients] is that I’ve run 2 trials…with it. Both are randomized, double-blind studies vs nivolumab as a single agent. I had a fellow in my clinic who was seeing a patient on the adjuvant trial,[and I challenged him to find out which treatment the patient was on based on toxicity presentation]. He told me he wasn’t sure he could do that and that’s the point. If we were treating the patient with ipilimumab/nivolumab, you would know which arm. If it was blinded and [using] ipilimumab/nivolumab, you would know which arm, but because it’s nivolumab/relatlimab, it was impossible to tell.
Efficacy of Relatlimab in Multiple Lines of Therapy
The phase 1 [portion of the] study had about 25 patients treated with [relatlimab as a] single agent in the second line, and it had no activity, but we only use it in combination because of the way it works; it has a lot more potential for working only in combination because it potentiates the TCR signaling. So once you use a PD-1 [inhibitor], you increase the TCR signaling, and then the LAG3 amplifies that signal and makes it better.
If you have an immune-suppressed individual who is already [being treated in the] second line and [is] resistant to immunotherapy, the TCR signaling is going to be so much more limited, and you’ll not [be] able to reverse the exhaustion with either single agent, whereas with a combination, you get about a 13% response in the second line [From the Data5]. Now, 13% [is a smaller response] and 1 out of 7 patients responded, so I’m not surprised that some patients feel like it never works. Every time I’ve used it in the metastatic [setting] in the second or third line, I’m just candid with patients [and I discuss how much of a response] I expect. I don’t do 3 months in that situation.… I just repeat their scans in 2 months because if they are going to progress I may want to do something different.
Adjusting Treatment
The data that we have [show] that if the patient is requiring steroids at the time of initiation, when you’re starting ipilimumab and nivolumab, their chances of a response are only 18%, so it’s limited. If you have already treated them with ipilimumab and nivolumab, and now you’re treating the toxicity with steroids…I would focus on finishing the steroids— completely tapering them off if you can—and then…consider rechallenging.
In CheckMate 067, the study that I ran in that population, if you had a grade 3 to 4 toxicity, they basically never rechallenged you with immunotherapy. They just took you off. We allowed [rechallenging] on the study after they taper off steroids…and we got away with it about half the time. The other half…would get hepatitis back or other things back, but you can get away with a rechallenge about half the time.
KEYNOTE-006 [NCT01866319], which compared 2 doses of pembrolizumab with single agent ipilimumab,…is interesting because we used to dose [patients] so high, [at about] 10 mg/kg every 2 weeks.8 The 200 mg that you currently use every 3 weeks is equivalent to 3 mg/kg every 3 weeks, so imagine how much more of a dose that was. And it didn’t matter, so there’s not a lot of dose-response relationship with pembrolizumab. By even decreasing the dose by almost twothirds, you still get the same outcome.
References
1. NCCN. ClinicalPractice Guidelines in Oncology. Melanoma, version 1.2023. Accessed March 2, 2023.https://bit.ly/3ILUa0B
2. Long GV, Robert C, Butler MO,et al. Standard-dose pembrolizumab plus alternate-dose ipilimumab in advancedmelanoma: KEYNOTE-029cohort1C, a phase 2 randomized study of two dosing schedules. Clin Cancer Res. 2021;27(19):5280-5288. doi:10.1158/1078-0432.CCR-21-0793
3. Opdualag Becomes First FDA-approved immunotherapy to targetLAG-3. National Cancer Institute. April 6, 2022.Accessed March 2, 2023. https://bit.ly/3kPrtI2
4. Amaria RN, Postow M, Burton EM,et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature. 2022;611(7934):155-160. doi:10.1038/s41586-022-05368-8
5. Tawbi HA, Schadendorf D, Lipson EJ,et al; RELATIVITY-047 Investigators. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24-34. doi:10.1056/NEJMoa2109970
6. FDA approves Opdualag for unresectable or metastatic melanoma. FDA. March 21, 2022. Accessed: March 2, 2023.https://bit.ly/3ERHJPz
7. Dimitriou F, Hauschild A, Mehnert JM, Long GV. Doubletrouble: immunotherapy doublets in melanoma-approved and novel combinationsto optimize treatment in advanced melanoma. Am Soc Clin Oncol Educ Book. 2022;42:1-22.
doi:10.1200/EDBK_351123
8. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239-1251. doi:10.1016/S1470-2045(19)30388-2