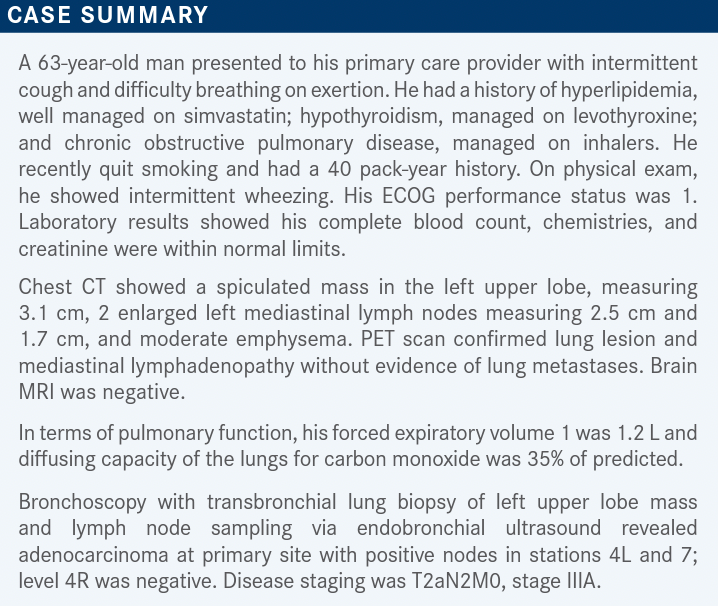
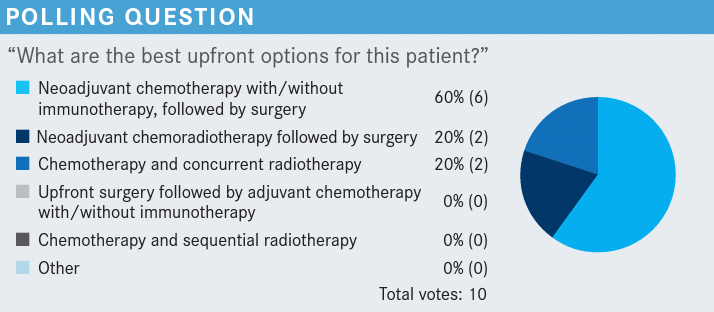
Roundtable Discussion: Kim Reviews the Use of Chemoradiation and Immunotherapy in NSCLC
During a Targeted Oncology™ Case-Based Roundtable™, Edward S. Kim, MD, MBA, discussed with participants utilizing chemoradiation and immunotherapy for patients with non–small cell lung cancer.

CHO: I don’t usually treat lung cancer, but when I used to do it, I’d typically use platinum therapy with paclitaxel [Taxol], with chemoradiation. And I know that there is immunotherapy you can combine with [chemotherapy] as a neoadjuvant therapy.
FU: You can use carboplatin plus paclitaxel or carboplatin plus pemetrexed [Alimta] with nivolumab [Opdivo]. And for the chemoradiotherapy, I use cisplatin with p16 [-positive disease]. I still remember 10 or 15 years ago [when] Dr Kim gave the first talk about that regimen. We have to do that before we decide on the immunotherapy. I also like to test for [PD-L1 expression] because I believe the [patients with] high PD-L1 probably benefited the most from this approach. [I have] no challenges or barrier to testing. We do the NGS testing and liquid biopsy testing if there is not enough tissue available.
KIM: Are you ordering NGS testing for all patients? For all stages? Or just selectively?
FU: I think before long you are going to have to order for all stages. Now we even have an adjuvant [EGFR-targeted] option, osimertinib [Tagrisso].
KIM: Yes, a lot of things are changing.

CHANDURI: We have started ordering [NGS testing] only recently. Otherwise, the common practice used to be to give carboplatin and paclitaxel weekly, along with radiation therapy, and see if the patient is able to go for surgery or not. Sometimes they might not be able to go for surgery because the cancer still [would be] stage IIIA, and after 4 cycles [we would do a] mediastinoscopy and see the response. Otherwise, NGS was not a common practice in early lung cancer.
KIM: Thanks, Dr Chanduri, for sharing that.

KIM: The patient has now finished chemoradiation. There has been a restaging CT scan. What is our approach now? Are you thinking about consolidation chemotherapy? Or are you thinking about giving durvalumab? What if the patient got worse and had weight loss? What’s the next step there?
CHANDAR: I would typically proceed to [consolidation] durvalumab at this point. I haven’t used a lot of consolidation chemotherapy in this setting. And if there were a borderline performance status, I don’t know that that would impact my decision for immunotherapy very much, unless the patient has some underlying autoimmune issues or something where I was really concerned about an immune-related adverse event. Most patients are able to tolerate durvalumab.
KIM: That’s been my experience, too.
FARJAMI: We are usually trying to follow the specific clinical trial [PACIFIC; NCT02125461] that led to the approval,1 and I think that we would wait for a follow-up restaging to confirm the response and [lack of] disease progression and then proceed with consolidation with immunotherapy.
KIM: That’s what I would expect out of you. Perfect.

FU: I usually would use durvalumab as a consolidation unless the patient has severe pneumonitis. [If so,] I would wait until the pneumonitis subsided, and then I would try to start. This patient has PD-L1 [expression of] 1%, right?
KIM: Yes. I will tell you that the PACIFIC study was independent of PD-L1 status. When you look at the subsets, the [patients with] low-to-zero [PD-L1 expression experienced] less of an effect, but we still treat them [From the Data2].
CHANDURI: Do you usually start durvalumab within day 14 after completion of the radiation therapy? Is that the recommendation?

KIM: To your point, the study [looked at the time period] between 1 and 42 days. And the subset analysis showed that the earlier you started durvalumab, [the better] it was. So, yes, if you are thinking 14 days, that’s a great number. The study did allow up to 42 days, but the subsets definitely favored early starting of durvalumab.3
CHANDURI: If you tested a patient and they were EGFR-mutation positive, what would you do? Would you continue with the durvalumab?
KIM: These are [instances] that you would probably want to avoid, [that is, patients with] any EGFR mutation. There have been some data that show [durvalumab and osimertinib are] not a good mixture.4
FU: In that case, would you do the tyrosine kinase inhibitor [TKI] plus radiation therapy?
KIM: We have no right answer yet in that setting. It is very tempting to do that, and I can tell you that when I discuss this with my colleagues, we always debate that. But there are no data to suggest it will help. You are looking at the future. We always have to do that because clinical trials are done today and the results are reported later. I think most of us still wait, and we know that patients who have EGFR mutations generally have a better prognosis overall, and if the tumor recurs or progresses during the waiting [period], I would still recommend a repeat biomarker testing at that point with a biopsy. And as we know, sometimes the EGFR mutation is preserved, and sometimes it’s not. So that’s generally the approach that I would take. But the question is a very pertinent research question that we are talking about.
FU: I can accept what you said, that the data [for patients with a PD-L1 level of less than 1%] are not [conclusive] because [those data represent] a small number of patients.3 But at least [there is room for] doubt. You may think that the patient will not benefit, but I can go with the FDA indication.5 But I have a [concern] with patients who are EGFR-mutation positive. There are data that show it’s actually detrimental [for those patients], especially after a patient has received radiation; there’s an increased risk for pneumonitis.4
KIM: I’m in complete agreement with that. I think we avoid [using osimertinib] on a patient like this who has an EGFR mutation. I definitely wouldn’t give it, personally. And, as you say, if there were a larger study that wanted to test [subgroups defined by PD-L1 expression levels of] less than or greater than 1%, is that something that’s going to change things? We always like to err on the side of overtreatment, and that’s one of the aspects. The other aspect is that it wasn’t too long ago that we were still debating [whether] PD-L1 overexpression was a bad marker, and [tumor mutational burden] was supposed to be the solution for all things [immunotherapy-related]. And it has kind of been the opposite. PD-L1 has stuck around, and tumor mutational burden hasn’t changed the world very much. So it’s interesting how things have shaped out.
FARJAMI: We try to test all patients [for] EGFR and ALK [mutations] and [for] the full panel. But one approach we started to adopt for [patients with] unresectable EGFR-positive disease, mainly stage IIIA [but also] stage I and II disease, is to start neoadjuvant therapy with osimertinib or one of the ALK-targeted therapies and convert the patients to the possibility of surgical resection. It depends on our institution and cardiothoracic surgeons. We have had some success with that approach with neoadjuvant targeted therapies and avoiding combining [modalities] for these patients, which takes us to a different path for these patients.
KIM: Those are great points. Over 10 years ago, we were in the era of just finding these EGFR mutations, but even before then, [we were seeing] very high response rates with TKIs. We have better ones now than we did back then. And it was so interesting. We were so hung up with neoadjuvant chemotherapy, right? And that was still [an] experimental [question] in lung cancer. And we concluded that it’s very hard to take a patient who is deemed unresectable up front and convert them to resection. Now, the situation you talked about was patients who are potentially resectable and making them even more resectable. There’s the nuance.
But I remember talking to Stephen G. Swisher, [MD,] and a few other [physicians] who were proposing neoadjuvant TKI treatment because you get a higher overall response rate in some of these patients of 60% to over 70%, as opposed to chemotherapy where you were probably getting 30% to 40% in a chemotherapy-naive neoadjuvant setting. Why wouldn’t you go with the more responsive [therapy]? And there was just this belief that it wasn’t as effective.
I think the future is definitely there. We know studies are being done to look at neoadjuvant treatment, not just with immunotherapies but also with TKIs and, hopefully, we will have that answer. Hopefully, we will enroll enough [patients] to the studies, and the studies will tell us that we can not only make the surgeries better with biologics, which we have seen with some data, but also convert patients from unresectable to resectable. That’s a threshold that’s been a little tougher to do in lung cancer.
FARJAMI: I think the same thing applies to some of these patients even with neoadjuvant [chemoimmunotherapy]. Also, I think there is space to have chemotherapy and immunotherapy along with radiation, with pembrolizumab [Keytruda]. With most of our patients, we try to [follow the conclusions of] the PACIFIC trial, but I think I have had cases in which I have used EGFR- and ALK-targeting drugs.
FU: I think, in practical terms, most of us are actually doing it [at times]. I had an 80-year-old nonsmoking White female patient. I…went ahead and tried the TKI. She lived more than 10 years, and she died of something else.
REFERENCES
1. FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC. FDA. February 16, 2018. Updated February 20, 2018. Accessed February 14, 2023. https://bit.ly/41iN0cJ
2. Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798-806. doi:10.1016/j.annonc.2020.03.287
3. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301-1311. doi:10.1200/JCO.21.01308
4. Aredo JV, Mambetsariev I, Hellyer JA, et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16(6):1030-1041. doi:10.1016/j.jtho.2021.01.1628
5. Imfinzi. Prescribing information. AstraZeneca; 2018. Accessed February 14, 2023. https://bit.ly/41kJO07