Munoz Covers Multiple Regimens for the Treatment of DLBCL
During a Targeted Oncology live virtual event, Javier L. Munoz, MD, MBA, discussed third-line treatments for DLBCL including chimeric antigen receptor T-cell therapy and targeted treatments.
Javier L. Munoz, MD, MBA
Director, Lymphoma Program
Mayo Clinic
Phoenix, AZ

Targeted OncologyTM: Based on your experience, is chimeric antigen receptor (CAR) T-cell therapy worthwhile for patients?
MUNOZ: The overall response rate [ORR] is somewhere between 50% and 80%.1-3 But what you care about in someone with an aggressive disease is a CR. [This means] no evidence of disease [and] everything that was abnormal before the CAR T cells becomes normal after the CAR T cells, whether it’s bone marrow involvement, a PET scan, or whatever that may be. CR rates are somewhere between 40% and 50%.1-3 Now, it’s tempting to look at the numbers and say, “Well, maybe one is a little better than the other.” I try not to do that because there’s [no] randomized trial comparing axi-cel [axicabtagene ciloleucel (Yescarta)] vs tisa-cel [tisagenlecleucel (Kymriah)] or liso-cel [lisocabtagene maraleucel (Breyanzi)]. So this is not apples vs oranges, but perhaps it’s a green apple vs a red apple.
They are not exactly the same, they’re a little bit different. I think the 3 of them work, the 3 of them have similar toxicities. [With] axi-cel, for example, the median overall survival has not been reached [in the ZUMA-1 trial (NCT02348216)].1 And when it comes to tisa-cel, [the median overall survival in JULIET (NCT02445248)] was 12 months.2 When it comes to liso-cel [in TRANSCEND-NHL-001 (NCT02631044)], it was 21.1 months.3 Again, this is the median, but if you truly look at the patients that achieved CR, you can see that the Kaplan-Meier curves plateau.
In the wording for the recent FDA approval for liso-cel, based on how the trial was designed, you will see some differences.4 For example, primary mediastinal lymphoma was allowed for liso-cel and it was also allowed for axi-cel.4,5 It was not part of the inclusion criteria for tisa-cel so that is why, for that particular construct, you should not prescribe tisa-cel, but this is just a reflection of how the trial was designed.6
What kind of toxicity do we see with these regimens?
Now, this is not a walk in the park. You can have severe toxicity, even lethality, and you could see fatalities. In the axi-cel trial of ZUMA-1, [slightly] more than 100 patients enrolled.1,7 Three patients suddenly had grade 5 toxicity; it’s a minority of patients but it can happen, and because of that you need to [counsel] the patients that there could be a lethal outcome. And when it comes to the toxicity, frameshift [seems to have occurred].
The first wave is cytokine storm. Then once that wave starts to get better—it’s not immediate, usually starts from day 2—it peaks around day 4 or 5 and then starts to trend down. Just when the patients are starting to get better from that, you start seeing, as I said,.…frameshift; you start seeing the second wave of toxicity, which would be neurologic toxicity.
We don’t understand why this happens because there’s no CD19 in the brain, for example. It’s probably interleukin mediated. You have this overwhelming amount of cytokines and interleukins that probably are going to get into your CSF [cerebrospinal fluid], and they are going to cause trouble. Now, what are the chances of having this severe cytokine storm? You have 2% for liso-cel and 11% for axi-cel.3 When it comes to neurologic toxicity, it’s 10% for liso-cel, 11% for tisa-cel, and 32% for axi-cel.1-3,7 Again, these are not randomized trials, so even though it’s tempting to look at those numbers and come up with strong conclusions, it always gives me pause to do that.
When it comes to CAR T cells, including managing the cytokine storm, you need to have at least 2 tocilizumab [Actemra] doses for each patient [for whom] you are going to prescribe CAR T cells. We’re facing a dosage shortage because [the drug was] also approved for COVID- 19. People are using tocilizumab left and right in these institutions that have CAR T cells and you don’t have enough for your patients receiving CAR T cells so that is causing problems.
I highlight [the issue of] prolonged cytopenias. [The regimen is] not for everyone; not every patient is going to go into CR. It’s almost like tossing a coin; it’s around 50%. So what happens for the patients that do not achieve remission and eventually progress? Sometimes they do have low counts, and that, in my clinic, is an obstacle to enrolling them in clinical trials subsequently. Those counts are still lingering a little bit on the low side, and that is challenging sometimes because they do not meet the inclusion criteria for the next trial you are thinking about.
Finally, if you prescribe a very strong anti-CD19 CAR T cell, you are going to cause B-cell aplasia. You’re going to obliterate the B cells, the normal B cells too, and this is going to cause low gamma globulins. These patients are going to be committed to IVIG [intravenous immunoglobulin] for life, and this is very expensive and is going to be troublesome for them.
We have seen that maybe 20% to 25% of patients require IVIG, and usually it’s for a brief period of time. Why? I think that is the poor man’s way…of looking at the persistence of the CAR T cells. It’s possible that they are not present for life, and as those CAR T cells start not being active anymore, your normal B cells start to come back, and that is why a patient that could need, briefly, IVIG, may not require it for life. Something that is documented in the trial, something that I have seen in my own personal practice, is that only a minority of patients post CAR T cells actually require IVIG. I would say most of them do not require it [long term], they require it for a brief period of time. Eventually the CAR T-cell persistence goes away and the normal B cells start to come back.
Do you need CAR T cells for life? I don’t know; I personally do not think so. I think you need the CAR T cells for as long as they do their job. If you have them floating around in your bloodstream for 6 months, and during those 6 months they obliterate all the cancer cells, good job. You do not need them for life. We know, based on the [National Institutes of Health] experience, that they have patients more than a decade after receiving CAR T cells [in whom] you cannot find persistent CAR T cells…they’re still in remission. They probably destroyed all the cancer cells during that brief period of time when the CAR T cells were active.
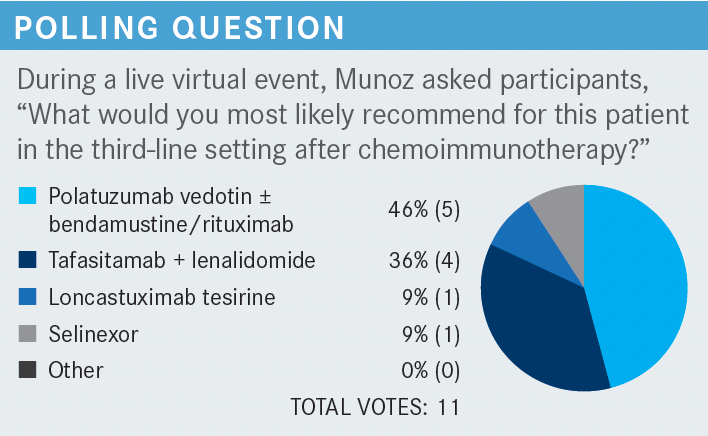
What do you think of the options for this patient?
Let’s talk about lonca-T [loncastuximab tesirine (Zynlonta)]. It’s important always to [look at] the verbiage of the label because this is what is going to allow you to prescribe it. To be fair and balanced, I’m going to discuss…all of the options so you [can decide…which one you would] want to choose with your patients. It’s an anti–CD19-directed antibody and [approved for] DLBCL, after 2 or more lines of systemic therapy, including DLBCL not otherwise specified, DLBCL arising from low-grade lymphoma, and also high-grade BCL.8 Lonca-T, in theory, is the one that is giving you that option, or at least it was studied in very few patients that have that double-hit and triple-hit lymphoma.
Again, this is an antibody-drug conjugate, similar to brentuximab vedotin [Adcetris] but brentuximab acts against CD30. Lonca-T is targeting CD19 and the payload is pyrrolobenzodiazepine for this particular agent. The trial [for lonca-T in relapsed/refractory DLBCL] was called LOTIS-2 [NCT03589469].9 Patients with 2 or more lines of [previous] systemic therapy [were enrolled] and needed to have CD19 positivity via biopsy, particularly prior anti-CD19 therapy, and this medication [was prescribed] as a 30-minute infusion every 3 weeks for up to a year. You want to pack a punch so you start with a little bit of a higher dose for the initial 2 cycles. After those initial 2 cycles, you go with a lower dose, and then you use a maintenance dose follow-up every 12 weeks for up to 3 years. The primary end point was ORR and they looked at many other variables.
Let’s look at the phenotype of the patients that enrolled. Age is something important: median was 66 years.9 The oldest patient was 71 years old and 10% of patients had double- or triple-hit lymphoma. And that is why this made it all the way to the label, allowing you to prescribe lonca-T in this population. Again, not a dramatic number of patients with double- or triple-hit [lymphoma] but there were some, and that is why you do have some evidence of seeing some improvement in this population.
The median number of prior systemic therapies was 3.9 The highest number was 4, so again, this population of patients was not heavily pretreated as we have seen in other patients. But I don’t think that they were cherry-picking, necessarily, because 58% of patients were refractory to the last line of therapy and 14% of patients had received a prior autologous stem cell transplant. Only 1 patient had received a prior allogeneic transplant.
What was the efficacy seen in LOTIS-2?
The primary end point was ORR—easy to remember because it’s 48% and that was half CR and half PR [95% CI, 39.9-56.7%].9 Every time I look at aggressive lymphomas, I try to zero in on CR because that is where I want to take those patients with aggressive disease. Median time to first response was 41 days so it’s not necessarily immediate, and the mean number of cycles that patients with lonca-T received was 4.5, so again, it’s not a therapy that will necessarily last forever. The minimum [number of cycles] was 1, in a patient that progressed quickly. The maximum that someone received was 18 cycles. Something [that should be] highlighted is whether or not [lonca-T is effective in patients who] have had a transplant before, [and] this medication seems to work even in high-risk subgroups.
The median duration of response was 13.4 months, so that is decent for a treatment that you could prescribe off the shelf in your clinics.9 The median duration of response for patients that achieve CR has not been reached, so that is pretty decent in a study that allowed patients that had high-risk features. About that controversy, if patients can receive subsequent therapies that also are attacking CD19, again, when you have a hammer, everything looks like a nail. So it’s very tempting, with so many anti- CD19 maneuvers, to keep just hammering down the CD19. [There’s] nothing surprising there; we have done that with the anti-CD19 [and] we have done that against CD20 for the longest time. After chemotherapy A fails, we use chemotherapy B, and we plug in another anti-CD19 or anti-CD20 treatment. So it’s not surprising to do this, and these companies are starting to generate some data to back up that story.
In 15 patients who received CD19 there are active CAR T cells after lonca-T, which isn’t anti-CD19 treatment, and they saw an ORR of 46.7% so [patients] can still respond.4 It seems that they can still respond to an anti-CD19 CAR T cell even if you have used an anti-CD19 treatment previously. Nine patients proceeded to stem cell transplant as consolidation after lonca-T.
How did patients do in terms of AEs related to lonca-T?
There was a little…atypical toxicity, something that is a little bit unique, which was peripheral edema.4 [Stratified by age group, the results in those younger] than 65 years… was 16.9% and in those older than 65, it was 22%. Overall, 20% of patients had peripheral edema. [Many of the most common treatment-related adverse events were hematologic in nature, which is] pretty much as expected, right?
These patients have received R-CHOP and/or R-GemOx before…so it’s not surprising that low counts are something that you are going to see commonly. The most commonly seen grade 3 or higher treatment-related adverse events, or treatment-emergent adverse events, were neutropenia, thrombocytopenia, and anemia, so no surprises there. [There was] no increased toxicity in patients older than 65, something for our population [that is] always very important. And for patients that started to develop that peripheral edema, of course,…that toxicity [was managed] with some diuretics.
Some practical information: It’s a 30-minute IV infusion.8 You start again every 3 weeks [and] you start with a higher dose. Subsequently, you step down to a lower dose. And it is recommended to give premedication, [in other words] steroids. [The prescribing information has] a nice algorithm or recommendation when it comes to decreasing the dose if you face severe cytopenias, which, again, seem to be the most commonly seen adverse events.
What is new and upcoming when it comes to lonca-T?
You have phase 3 trials trying to look at lonca-T plus rituximab vs R-GemOx [eg, LOTIS 5 (NCT04384484)]10 because again, in their calculation, R-GemOx is still a treatment that is very commonly used for DLBCL. And I think they are right; it’s something that is relatively easy to use and most of us are going to feel comfortable using R-GemOx, so they’re just trying to prove that lonca-T plus rituximab is better than R-GemOx. They’re going to have a part 1…a run-in initial part of the therapy that is going to be the evaluation of lonca-T plus rituximab. Again, it has not been done before so they need to do it that way. If they prove that lonca-T plus rituximab is safe, then you come in with part 2, which is…the randomization of lonca-T plus rituximab vs R-GemOx and a decent number of patients north of 300.
Multiple participants voted for tafasitamab (Monjuvi) plus lenalidomide (Revlimid). What is the background for using this combination?
[This combination is approved for use in patients with] DLBCL not always specified or DLBCL arising from a low-grade lymphoma, who are not eligible for an autologous stem cell transplant.11
Lenalidomide [acts as an] immunomodulator, [an anti- CD19 monoclonal antibody].11 It has activity in many diseases, [such as] myelodysplastic syndrome, multiple myeloma, and mantle cell lymphoma, so it’s not surprising that it has activity in DLBCL. The name of the trial that led to the approval was called L-MIND [NCT02399085], and it’s a phase 2 study.12
They were looking for patients that had received 1 to 3 prior lines of therapy…not eligible for high-dose chemotherapy and autologous stem cell transplant, and, just as [with] lonca-T, you start a little bit more aggressively. In cycle 1 to cycle 3 you have more infusions of tafasitamab. In cycle 4 to cycle 12, [you have the] same dose but you’re going to give it less frequently, only 2 infusions per month. Lenalidomide is a 25-mg/day dose. [From] day 1 to 21, sometimes, the higher the dose of lenalidomide the tougher the disease [can be] to tolerate. It is not a completely clean drug either. You have patients that can complain of skin rashes [and] diarrhea, [and] there’s a chance of having a clot, so that’s why I usually like to prescribe some sort of antithrombotic prophylaxis. I usually go with a baby aspirin [for] my patients [to whom] I prescribe lenalidomide.
Tumor flare is also something that has been described.11 When I put patients on lenalidomide, sometimes they call me saying, “What you gave me did not work.” The lymph node is just growing and growing quickly, and I tell them to come to the clinic. By the time they come to the clinic, the mass has receded; it just went away. It happens usually at the very beginning when you start lenalidomide and it’s called tumor flare. The lymphoma starts fighting that lenalidomide and that causes a little bit of worsening lymphadenopathy, but if you muscle through, the lymph node shrinks in size again.
And then if you face stable disease or higher, CR or PR, you can continue the tafasitamab without lenalidomide until progression, cycle 12 or more, and the primary end point in the trial was ORR.12 One of the things I always look at is age. The median age [in this study] was 72 and the oldest patient was 76 years old.12 Median prior lines of therapy are also important because if you are trying to compare trials, it’s not necessarily fair to compare one [in which] the median [number of prior] therapies was 2 vs 1 [in which it] was 4 or 5. But in this study it was 2 prior lines of therapy. [The percentage of patients who were] refractory to the last therapy was 44%, so again this is not necessarily cherry picking. [The percentage of patients who had a] prior transplant was 11%.
Describe the results from the L-MIND trial.
The ORR—again, the one I care about the most—would be the longest follow-up. In this case, more than 35 months after [treatment with] tafasitamab/lenalidomide, there was a 57.5% ORR.12 I care about the CR, which was 40%, and a median duration of response of 43.9 months, so [this is] really high for a maneuver that is not CAR T-cell based and you do not necessarily have to send the patient to another oncology center.
At a median follow-up of 33.9 months, the median progression-free survival is 11.6 months.12 So again, [this therapy is] not for everyone; you could prescribe it and [see] if there is progression of disease. Then, of course, we could still be happy to see your patients if you consider CAR T cells, but for the ones that do go into CR, they seem to have good options there. The median overall survival at a median of 42.7 months follow-up was 35.5 months.
If it is a safe drug, you’re not going to have a lot of people stopping it or discontinuing treatment because of the severe adverse events. Twelve percent stopped treatment due to toxicity, due to adverse events, and serious adverse events did occur in 51% of patients, so again, it’s not like drinking a glass of water.11,12 Treatment-emergent adverse events leading to death [were observed in] 13%, or 4 of 30 patients. There were patients with dose reductions; 45.7%...had at least 1 or more events of dose reduction, particularly of the lenalidomide. [There were some] grade 4 toxicities, such as neutropenia.11,12 If you look at tafasitamab monotherapy, you still see neutropenia, but definitely, with the addition of lenalidomide, [it is] higher all across the board when it comes to toxicity. So it seems that the combination of tafasitamab/ lenalidomide gives you more efficacy but it also gives you a little more toxicity.
What is the manufacturer of tafasitamab doing when it comes to future trials?
[There is the RE-MIND trial (NCT04150328), which is] looking at real-world data.13 [Investigators are evaluating] tafasitamab plus lenalidomide vs lenalidomide in transplant-ineligible patients with relapsed/refractory DLBCL, and the primary end point is ORR. If I had to choose or guess, tafasitamab/lenalidomide is going to do way better than lenalidomide monotherapy per se. I would only choose lenalidomide monotherapy in patients with DLBCL that have the activated B-cell [ABC] subtype instead of the germinal center subtype.
There are some data showing that [patients with] ABC seem to respond a little bit better to lenalidomide or even ibrutinib [Imbruvica]. Ibrutinib is not approved in DLBCL, but in clinical trials we have seen that the ABC subtype seems to work a little bit more when you expose those patients to Bruton tyrosine kinase inhibitors, compared with germinal center DLBCL.
Polatuzumab vedotin (Polivy) plus or minus bendamustine and rituximab (pola-BR) had the most votes in the earlier poll. What role does that regimen play in DLBCL?
This particular study [NCT02257567] was for DLBCL, but there was a cohort that also had follicular lymphoma. [The investigators] thought, in their calculation, that it was going to be a home run for follicular lymphoma and they were not sure about DLBCL.14 The numbers ended up looking better for DLBCL than follicular lymphoma, and that is why it’s not approved right now in follicular lymphoma. It’s approved in DLBCL.15
When it comes to the cohort for DLBCL—80 patients, bendamustine vs pola-BR—forget a little bit about the follicular lymphoma cohort because it was negative.14 It is not FDA approved in follicular lymphoma at this point and you have the exclusion criteria there. Prior allogeneic stem cell transplant was excluded, transformation of indolent disease to DLBCL was excluded, [and grade 2 or higher] neuropathy was excluded. The payload in polatuzumab is a vinca alkaloid.15 It causes neuropathy so it makes sense that you would be careful to include those patients.
In the pola-BR arm, the oldest age of patients enrolled was 86; in the bendamustine arm the oldest age…was 84 years.14 These were patients that had high IPI scores in [the pola-BR] arm, 55%, but in the bendamustine arm [it was] 72.5%, again highlighting the differences. It seems that patients in the bendamustine arm had higher IPI scores [than] the patients in the pola-BR arm, so that’s a difference that I identified.
The median prior lines of therapy was 2 for both, so I think that was fair.14 Refractory [disease was], numerically, a little bit higher with bendamustine and rituximab. Prior stem cell transplant [numbers were] a little bit higher with pola-BR. So, if you truly want to be the devil’s advocate, you could say you were selecting a little bit for tougher patients in the bendamustine and rituximab arm because [there were patients with] numerically higher refractory disease and numerically higher IPI in that particular cohort. Also, if more patients had received prior stem cell transplant in the pola-BR arm, maybe they had a better protoplasm or a better phenotype compared with the other arm.…But those numbers are numerically different but not dramatically different. Finally, there was a little bit more germinal center DLBCL in the bendamustine [vs the] rituximab arm, 42% vs 37%, [respectively].
How did patients respond to pola-BR in this trial?
The ORR was 45% for pola-BR, and you could see that for bendamustine and rituximab it was only 17.5%.14 The CR rate [was] 40%, so a reasonable number of patients achieved CR with polatuzumab; 40% is a good number. Remember what you get with CAR T cells—40% to 50%, depending on the construct.
[Regarding] progression-free survival, the median for pola-BR was 9.5 months vs 3.7 months if you only go with bendamustine and rituximab.14 Again, it’s not for everyone. It’s not necessarily a home run [and] probably not a destination therapy; it’s probably a breach, but you could get several months for your patient when it comes to putting them in remission—9.5 months. The overall survival had differences there, too, [approximately] a year. The overall survival was 2.4 months for pola-BR vs 4.7 months for bendamustine and rituximab.
Let’s take a look at the peripheral neuropathy: grade 3 or 4 was 0%, so that is good, but 43.6% had some degree of neuropathy so that will be grades 1 to 2.14 Forty-three percent is almost as [high as the percentage of patients who] achieved CR, which was 40%. Approximately 40% of patients will also develop peripheral neuropathy. When it comes to bendamustine and rituximab, not surprisingly, the number is way lower—7%. Neuropathy is not 1 of the most common things that you see with bendamustine and rituximab.
REFERENCES
1. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. doi:10.1016/S1470-2045(18)30864-7
2. Schuster SJ, Bishop MR, Tam CS, et al; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. doi:10.1056/NEJMoa1804980
3. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. doi:10.1016/ S0140-6736(20)31366-0
4. Breyanzi. Prescribing information. Juno Therapeutics Inc; 2021. Accessed January 12, 2022. https://bit.ly/33AuXWa
5. Yescarta. Prescribing information. Kite Pharma, Inc; 2021. Accessed January 12, 2022. https://bit.ly/3Gxi11A
6. Kymriah. Prescribing information. Novartis Pharmaceuticals Corp; 2021. Accessed January 12, 2022. https://bit.ly/3qIcuz
7. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531- 2544. doi:10.1056/NEJMoa1707447
8. Zynlonta. Prescribing information. ADC Therapeutics SA; 2021. Accessed January 12, 2022. https://bit.ly/3Gxidhk
9. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:10.1016/ S1470-2045(21)00139-X
10. Hamadani M, Linhares Y, Gandhi M, et al. Phase 3 randomized study of loncastuximab tesirine plus rituximab versus immunochemotherapy in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL): LOTIS-5. J Clin Oncol. 2021;39(suppl 15):TPS7574. doi:10.1200/JCO.2021.39.15_suppl. TPS7574
11. Monjuvi. Prescribing information. MorphoSys US Inc; 2021. Accessed January 12, 2022. https://bit.ly/33DASty
12. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
13. Zinzani PL, Rodgers T, Marino D, et al. RE-MIND: comparing tafasitamab + lenalidomide (L-MIND) with a real-world lenalidomide monotherapy cohort in relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2021;27(22):6124-6134. doi:10.1158/1078-0432.CCR-21-1471
14. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
15. Polivy. Prescribing information. Genentech, Inc; 2020. Accessed January 12, 2022. https://bit.ly/3fsjeeI
