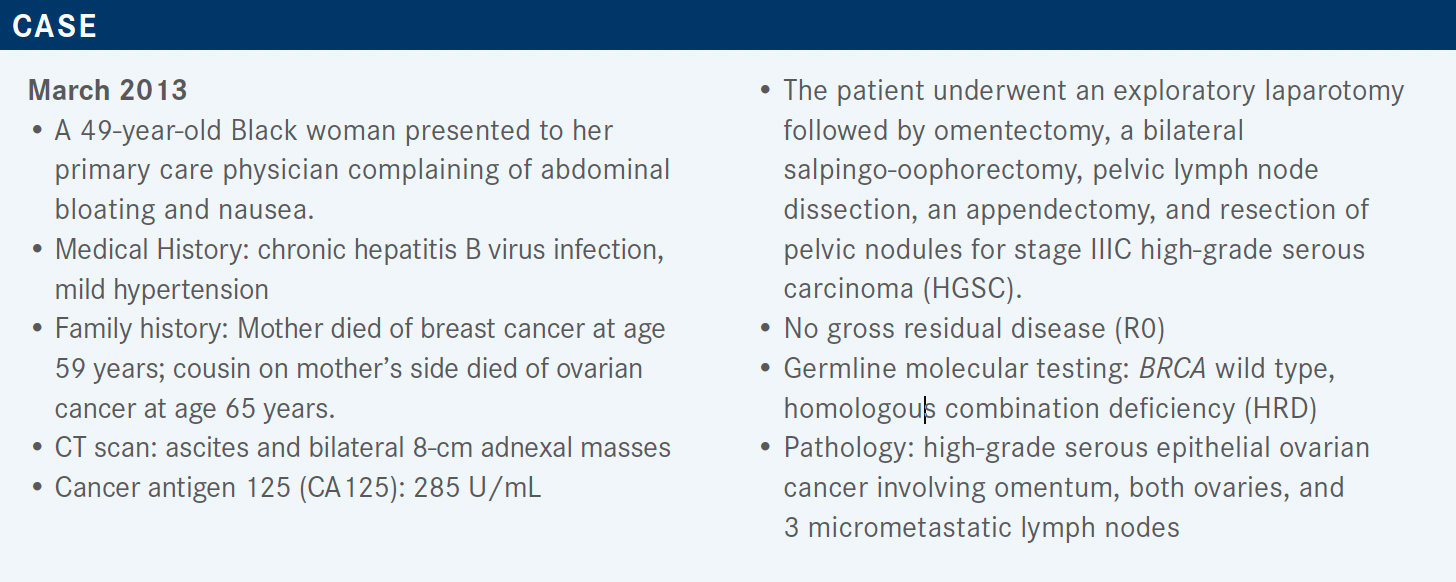
Grisham Discusses Use of Multiple PARP Inhibitors in Treating Ovarian Cancer
During a Targeted Oncology case-based roundtable event, Rachel N. Grisham, MD, discussed clinical trials of different PARP inhibitors for patients with ovarian cancer.
Rachel N. Grisham, MD
Section Head, Ovarian Cancer
Director, Gynecologic Medical Oncology
Memorial Sloan Kettering Westchester

Targeted OncologyTM: What factors do you consider when choosing a first-line maintenance regimen for patients with ovarian cancer?
GRISHAM: [Data from] recent studies, and multiple new FDA approvals, have led to a change in how we treat our patients in the upfront setting and [this is reflected in] the National Comprehensive Care Network [NCCN] guidelines.1
The main point of these guidelines is that we should do molecular testing in all of our patients at the time of diagnosis and then consider those results when we [choose] first-line maintenance treatment options because we now have multiple FDA-approved options.
For all our patients with a BRCA mutation [in their tumor], it is recommended to give a PARP inhibitor if they have advanced-stage disease and have responded to chemotherapy.2,3 For those patients who received bevacizumab [Avastin] in the front-line setting [and are] positive for HRD, it’s generally recommended to give the combination of bevacizumab with [the PARP inhibitor] olaparib [Lynparza]2; if they are HRD negative, we consider just continuing the bevacizumab by itself.1 A lot of thought and discussion with the patient has to go into making these individual [treatment] decisions, which are based on a patient’s tolerance of their initial chemotherapy, their expected tolerance of the PARP inhibitors, and their individual molecular profile.
What data support the use of olaparib as maintenance therapy after first-line chemotherapy?
SOLO-1 [NCT01844986] is the phase 3 study [whose findings] led to the initial FDA approval of olaparib as a maintenance therapy for patients with a germline or somatic BRCA mutation following response to initial surgery and chemotherapy.4,5 This study enrolled patients who had a BRCA mutation; it could have been germline or somatic but virtually all the patients enrolled in the study had germline mutations because that’s how we were testing back then.
The patients had newly diagnosed ovarian cancer and had received prior platinum-based chemotherapy. They were randomly assigned in a 2:1 ratio to receive either olaparib or a placebo, respectively, with investigator-assessed progression-free survival [PFS] being the primary end point.4 We now have more than 5 years of follow-up from this study, and the fantastic results were presented at the ESMO [European Society for Medical Oncology] Congress in 2020, which showed continued PFS benefit.
Recurrence occurred in only half the patients treated with olaparib vs in almost 80% of patients treated with placebo, at a median PFS of 56.0 months vs 13.8 months, respectively [HR, 0.33; 95% CI, 0.25- 0.43].6 Olaparib maintenance also produced a good duration of response. It is FDA approved for these patients and we strongly recommend that all our patients with a germline or somatic BRCA mutation have front-line maintenance with a PARP inhibitor.5
Additionally, all the subgroups benefited from the use of olaparib. These subgroups were defined by either a complete or partial response to chemotherapy, ECOG performance status, age, BRCA mutation status—either BRCA1 or BRCA2—and other criteria as well.4 [These results suggest that] all patients with a BRCA mutation in their tumor are going to benefit from the use of a PARP inhibitor in the frontline maintenance setting. After 5 years of follow-up, the safety profile remained consistent and no additional cases of myelodysplastic syndrome [MDS] or acute myeloid leukemia [AML] were reported.
The incidence of new primary malignancies was balanced between arms. I know we see different levels of MDS and AML in different studies, and some studies show higher rates of MDS and AML in patients who received more lines of chemotherapy, but I did find these data reassuring. Among patients treated with olaparib, common adverse events [AEs] included nausea and [other] gastrointestinal [GI] AEs such as fatigue, headache, and abdominal pain.4,6 We’re often able to mitigate [nausea-related] AEs with the use of anti-nausea medications.
Niraparib [Zejula] is another PARP inhibitor. What data support its use in patients with high-risk ovarian cancer?
The PRIMA trial [NCT02655016] examined niraparib vs placebo in the high-risk population of patients who had newly diagnosed ovarian cancer and had received neoadjuvant chemotherapy, or had stage IV disease, or had residual disease after debulking for stage III disease. Patients who were stage III and who had a complete gross resection were not eligible. This study looked at all comers, unlike the SOLO-1 trial in which all patients had a BRCA mutation. The study was a double-blind, randomized, placebo-controlled phase 3 study and looked at patients who had achieved a complete response or a partial response following their first-line platinum chemotherapy. Patients were randomly assigned in a 2:1 ratio to receive either niraparib or placebo, respectively.7,8
There was an amendment during this study to allow for a modified dosing schedule. Initially, all patients received 300 mg daily. Later, the study was amended to allow for a 200-mg starting dose for patients with lower platelet counts or lower body weight at the start of treatment. Similar results were found between those patients treated with the higher starting dose and the patients treated with the individualized starting dose.
The primary end point for the PRIMA study was PFS in all comers, regardless of BRCA mutation status and regardless of HRD status. There was an improvement in PFS at 13.8 months with the use of niraparib maintenance therapy vs 8.2 months with placebo, with an HR of 0.62 [95% CI, 0.50-0.76].7 This led to the FDA approval of niraparib for all patients, regardless of molecular status, in the front-line maintenance setting.9
The subgroup analysis showed that patients who had a BRCA mutation derived the greatest PFS benefit from the addition of the PARP inhibitor vs placebo [HRD positive with BRCA mutation: HR, 0.40; 95% CI, 0.27-0.62]. There was a lesser benefit for patients who had no BRCA mutation [HRD positive with no BRCA mutation: HR, 0.50; 95% CI, 0.31-0.83]. The smallest benefit was observed in patients who were HRD negative [HR, 0.68; 95% CI, 0.49-0.94]. All other subgroups, including those defined by age, disease stage, and response to chemotherapy, also showed benefit with the PARP inhibitor.7
An interim analysis showed that overall survival [OS] numerically favored niraparib over placebo, but keep in mind that definitive conclusions cannot be drawn because OS event rates are low. This will be updated once the data are more mature.10
The toxicity profile is like that of olaparib, generally manageable with the use of conservative measures, supportive care, and dose reductions as needed, in addition to the individualized starting dose. Treatment discontinuation due to thrombocytopenia was relatively low, occurring in only 4.3% of patients, and dose interruptions were [similar to] those in previous niraparib trials.11
[As we would expect], there were more hematologic AEs [among patients who received niraparib]. I generally think of olaparib as causing more GI AEs and niraparib as causing more hematologic AEs, particularly thrombocytopenia, which can be severe, and anemia, [both of which were observed in the experimental arm of this study].
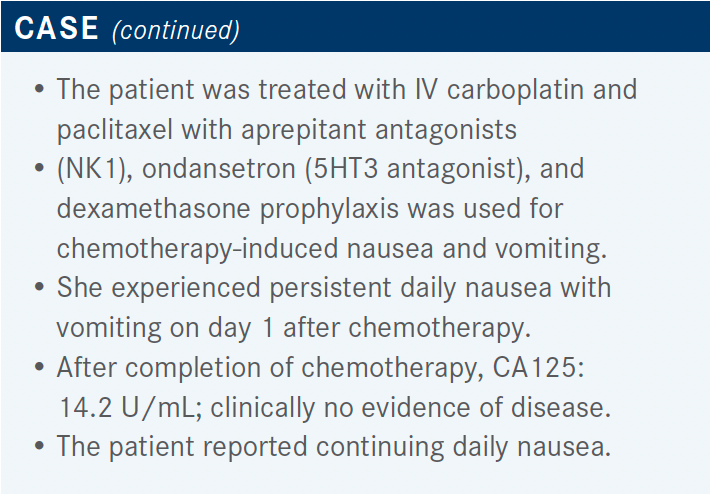
Has either niraparib or olaparib been examined in combination with any other treatment as part of a maintenance regimen?
The PAOLA-1 study [NCT02477644] was a phase 3 study that examined the combination of bevacizumab plus olaparib vs bevacizumab plus placebo as maintenance therapies. Patients in this study had newly diagnosed advanced ovarian cancer. They had completed their first-line surgery and platinum-based chemotherapy plus bevacizumab, and then had either a complete or partial response to their initial treatment. Patients in this study were allowed to have had neoadjuvant chemotherapy, like patients in the PRIMA study, and could not have had prior PARP inhibitor therapy. In this study, olaparib was administered at a dosage of 300 mg twice a day.12
Overall, the patients who received bevacizumab plus olaparib had an improved PFS vs patients who received bevacizumab plus placebo [HR, 0.59; 95% CI, 0.49-0.72; P < .001]. This benefit was more pronounced in those patients who had BRCA mutations and in those patients who were HRD positive. Patients who were HRD negative [showed no benefit from receiving the experimental combination].15 For both the PRIMA study and….PAOLA-1 study, HRD status was determined using the Myriad myChoice [CDx] assay to look for HRD, with a cutoff score of 42.11,14
In the PAOLA-1 study, patients experienced the AEs that we would expect from olaparib—nausea, fatigue, and some decrease in cell counts. [There were also] some bevacizumab AEs, including hypertension, but overall the AEs were manageable.13
What trends in toxicity were observed among these studies?
You can’t directly compare the results of the different trials, of course, because the studies involved different populations of people. [However,] anemia and thrombocytopenia were observed more [often] in the PRIMA trial of niraparib than in the other 2 studies. Nausea and fatigue were observed more [often] in the SOLO-1 trial of olaparib, and for each of these AEs, the percentage of affected patients was similar between the other 2 studies.2,3,13 We generally [associate] hematologic AEs with niraparib and GI AEs with olaparib.
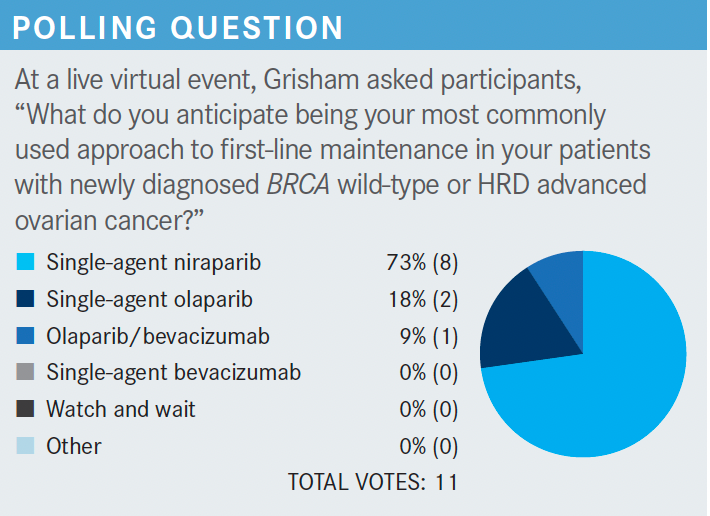
What dose adjustments are recommended to maximize the tolerability of these drugs?
[Dose adjustment] is important, I think, in making niraparib more tolerable. In the PRIMA study, initially, niraparib was given to all patients at a standard dosage of 300 mg once a day.
However, as previously mentioned, the study was amended to incorporate a patient-specific dosage based on the patient’s body weight and platelet count to make the regimen more tolerable. The results obtained with each dosage were similar.3,7,8
When starting niraparib, I always look at a patient’s weight and platelet count. First, I give the patient time, usually 4 to 6 weeks, to recover their platelet count after chemotherapy.
Then, if their body weight is 77 kg or greater and if they have a platelet count of 150,000/μL or higher, I start them at the full dose of 300 mg once a day. If their weight is less than 77 kg, which isn’t that low for shorter people, or if their platelet count is less than 150,000/μL, then the individual starting dose is 200 mg once a day. Using an individualized starting dose can [improve] the tolerability of niraparib and prevent [later] dose reductions necessitated by decreased platelets.3,16
Dose adjustments are also available for both olaparib and niraparib based on AEs such as persistent fatigue, persistent nausea despite medical management, and persistently decreased platelet counts despite individualized dosage. It’s a little more complicated to change the dosage of olaparib than that of niraparib, because in the case of olaparib you have to get prescriptions for new pills.
Olaparib’s starting dosage is 300 mg twice a day, and patients take two 150 mg pills per dose. If you want to do a dose reduction, the first dose reduction is to 250 mg twice a day; each dose consists of a 100 mg tablet and a 150 mg tablet, so you do have to submit a new prescription for the 100 mg tablets. The second dose reduction is to 200 mg twice a day, using the 100 mg tablets. It is [a nuisance] to submit that new prescription but it is best to use this stepwise dose reduction for olaparib.2
Reducing the dose of niraparib is a little more straightforward. If the patient weighs 77 kg or more and has a platelet count of 150,000/μL or higher, you start them at the full dosage of 300 mg once a day. I usually tell my patients to take it in the evening, then they can have a dose reduction to 200 mg or 100 mg. If they start at a 200-mg dose, they can have a dose reduction to 100 mg, but after that, if [the drug is] not tolerated, I discontinue it.3
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2022. Accessed January 18, 2022. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
2. Lynparza. Prescribing information. AstraZeneca Pharmaceuticals LP; 2021. Accessed January 20, 2022. https://bit.ly/3HBpE83
3. Zejula. Prescribing information. GlaxoSmithKline; 2021. Accessed January 20, 2022. https://bit.ly/3Jdt40Z
4. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505. doi:10.1056/NEJMoa1810858
5. FDA approved olaparib (LYNPARZA, AstraZeneca Pharmaceuticals LP) for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated (gBRCAm or sBRCAm) advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based. FDA. December 19, 2018. Updated December 26, 2018. Accessed January 20, 2022. https://bit.ly/3gzmlCa
6. Banerjee S, Moore, KN, Colombo N, et al. Maintenance olaparib for patients (pts) with newly diagnosed, advanced ovarian cancer (OC) and a BRCA mutation (BRCAm): 5-year (y) follow-up (f/u) from SOLO-1. Ann Oncol. 2020;31(suppl 4):S613. doi:10.1016/j.annonc.2020.08.950
7. González-Martín A, Pothuri B, Vergote I, et al; PRIMA/ENGOT-OV26/ GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019:381(25):2391-2402. doi:10.1056/NEJMoa1910962
8. Monk BJ, Mirza MR, Vergote I, et al. A prospective evaluation of tolerability of niraparib dosing based upon baseline body weight and platelet count: blinded pooled interim safety data from the ENGOT-OV26/ PRIMA study. Gynecol Oncol. 2019;154(suppl 1):3-4. doi:10.1016/j. ygyno.2019.04.018
9. FDA approves niraparib for first-line maintenance of advanced ovarian cancer. FDA. April 29, 2020. Accessed January 20, 2022. https://bit. ly/3gyBGD2
10. Han SN, Monk BJ, González-Martín A. Time to first subsequent therapy (TFST) and progression-free survival 2 (PFS2) from the phase 3 randomized, double-blind PRIMA/ENGOT-OV26/GOG-3012 study in patients with newly diagnosed ovarian cancer. Gynecol Oncol. 2020;159(suppl 1):18-19. doi:10.1016/j.ygyno.2020.06.035
11. González-Martín A, Pothuri B, Vergote I, et al. Niraparib therapy in patients with newly diagnosed advanced ovarian cancer (PRIMA/ ENGOT-OV26/GOG-3012 study). Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394
12. PAOLA-1: platine, Avastin and OLAparib in 1st line. Paola-1-study. Accessed January 20, 2022. https://bit.ly/3stbyiq
13. Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416-2428. doi:10.1056/NEJMoa1911361
14. Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018 Aug 1;29(8):1784-1792. doi: 10.1093/annonc/mdy181. Erratum in: Ann Oncol. 2019 May 1;30(5):859.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More