Zain Evaluates Studies Offering Improvement in R/R DLBCL Survival
During a case-based rountable event, Jasmine Zain, MD of City of Hope, discussed the case of a 79-year-old patients with relapsed or refractory diffuse large B-cell lymphoma.
Jasmine Zain, MD
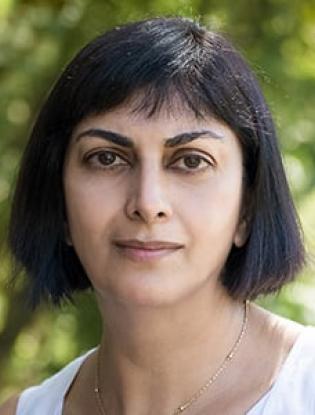
Targeted Oncology™: What are the second-line therapy options for transplant-ineligible patients with DLBCL?
ZAIN: For second-line therapy for DLBCL, we try to see [whether] the patient is potentially a transplant candidate or intends to proceed to transplant. If so, the standard cytotoxic chemotherapy salvage regimens can be offered to the patient.
However, if the patient is not a transplant candidate, thankfully we have a lot of new options. There is gemcitabine, oxaliplatin, with or without rituximab. We have polatuzumab [Polivy], which is now approved in combination with bendamustine [Treanda] and rituximab. There are some other newer regimens including tafasitamab [Monjuvi] and lenalidomide [Revlimid]. Brentuximab vedotin [Adcetris] can be used if the patient has CD30-positive disease. If the patient is being considered for chimeric antigen receptor [CAR] T-cell therapy, then some salvage bridging regimens can be considered provided the patient needs it.1
Because we are doing well with the patients [with DLBCL], we can talk about second[-line] and third-line therapies in case they relapse. A lot of the CAR T-cell therapies come into the third line of subsequent therapy options. Allogenic hematopoietic stem cell transplant [SCT] is considered as consolidation after second-line therapy. Selinexor [Xpovio] is another agent that is approved after 2 lines of systemic therapy in patients who have progressed even after transplant or CAR T-cell therapy, and there is also loncastuximab tesirine [Zynlonta].1
The problem is that some of these therapies, like tafasitamab or loncastuximab, are directed toward CD19 and CAR T-cell therapy is also directed toward CD19. It is unclear how these interact with each other. Pretreatment with a CD19-directed therapy could have a negative impact on the efficacy of subsequent anti-CD19 CAR T-cell therapy.
What data support the use of polatuzumab vedotin in relapsed or refractory DLBCL?
The study that led to the approval of polatuzumab vedotin, which is a CD79b-directed antibody-drug conjugate [ADC], was a phase 2 study [NCT02257567] that had polatuzumab in combination with bendamustine and rituximab [pola-BR]. The inclusion criteria were straightforward. [Patients had to be aged] over 18 [years], have biopsy-proven relapsed or refractory DLBCL, more than 1 line of prior therapy, their ECOG performance status had to be good, and the patient had to have minimal peripheral neuropathy because one of the adverse effects [AEs] of polatuzumab is neuropathy. The patient also had to be transplant ineligible or [must have] failed a prior transplant.2
The study was designed as a comparator study. Initially, there was a safety run-in [period] of pola-BR with 6 patients. Following that, the patients were randomized either to pola- BR or BR alone with 40 patients in each arm. Then there was an expansion cohort of another 106 patients on pola-BR. A total of 152 patients were eventually evaluated.2
Polatuzumab vedotin was given at 1.8 mg/kg daily and bendamustine dosing was at 90 mg/m2 for 2 days. The rituximab was repeated every 3 weeks and treatment was a 21-day cycle for a total of 6 cycles. The primary end point of complete response [CR] was determined by PET.2 The results were such that the FDA gave an accelerated approval on June 10, 2019, for pola-BR in DLBCL after patients failed 2 lines of therapy.3
The patients were fairly matched in the pola-BR, BR, and pooled BR arms. The median age was 67 years, but a little bit older in the BR arm, and they tried to balance the risk categories. There were 45% of patients in the pola-BR arm and 27.5% in the BR arm with an IPI of 0 to 2, and 55% and 72.5%, respectively, with an IPI of 3 to 4. Patients with stage III or [stage] IV disease [comprised] 85% of the pola-BR arm and 90% of the BR arm. The median number of lines of therapy was 2 in both arms, but the range was a little bit higher in the pola-BR arm at 1 to 7, whereas the BR arm [ranged from] 1 to 5.2,4
The duration of the last treatment was less than 12 months in almost 80% of patients in all 3 cohorts, and 75% to 85% of patients were refractory to prior lines of therapy. Prior SCT was not very common. It was 25%, 15%, and 18% in the pola-BR, BR, and pooled BR arms, respectively. The GCB subtype in the 3 arms ranged from 37% to 42%. The median time to first response was 2 months.2,4
The pola-BR arm had 40 patients and the overall response rate [ORR] was 45% with 40% being CRs. The BR arm had a 17.5% CR rate and the expansion cohort had a 38.7% CR rate with an ORR of 41.5%.2,4 The median duration of response [DOR] in the pola-BR arm was 10.9 months, the BR arm was 10.6 months, and the extension cohort was 9.5 months.
It did not look like there was much of an advantage, but the median progression-free survival [PFS] showed a difference that was statistically significant. It was 9.2 months in the pola-BR arm vs 3.7 months in the BR arm and 6.6 months in the extension cohort.4 An overall survival [OS] benefit was also seen, which was a surprise. It was 12.4 months for the pola-BR arm vs 4.7 months for the BR arm. This led to the FDA approval of this combination.3
There was a discontinuation rate due to AEs that was higher in the pola-BR arm at 33% vs 10% in the BR arm. There were some dose reductions seen as well in both arms. All grades of hematologic toxicity were a little bit higher in the pola-BR arm, but febrile neutropenia was pretty similar in both arms.
Diarrhea, nausea, and gastrointestinal issues were similar too with all grades of diarrhea at [38.5% for pola-BR and 28.2% for BR]. Fatigue and pyrexia were similar. All grades of peripheral neuropathy [totaled] 43.6% for pola-BR vs only 7% for BR.2 This was attributed mostly to polatuzumab.
What data support the use of tafasitamab plus lenalidomide in relapsed or refractory DLBCL?
Tafasitamab is a CD19-directed monoclonal antibody. It is produced by MorphoSys AG. It works on tumor-targeted cells by causing antibody-dependent cellular cytotoxicity and phagocytosis. It also causes direct cell death. There was single-agent activity that was encouraging in relapsed or refractory DLBCL.5
We do not know [for certain] how lenalidomide works, but it is thought to increase T-cell and NK [natural killer]– cell activation and expansion. There is also some direct cell death activity, and it has been approved for multiple types of lymphoma.
Tafasitamab plus lenalidomide was studied in vivo and in vitro and the combination seemed to have synergy, so it was studied in relapsed or refractory DLBCL. Eventually, that led to its approval on July 31, 2020, for patients with relapsed or refractory DLBCL, including DLBCL that arises from low-grade lymphoma.6 But it is only approved for patients who are not eligible for autologous SCT based on the study design.
The phase 2 L-MIND study [NCT02399085] led to the approval of this combination. It was an open-label, multicenter study. The eligibility criteria were reflected in the final label of the combination. Patients had relapsed/ refractory DLBCL with at least 1 to 3 prior lines of therapy. Patients had to be ineligible for high-dose therapy or autologous SCT, which was by design. They also excluded primary refractory patients.7
The schedule is a little complicated for the first 3 cycles. Tafasitamab is given at 12 mg/kg for a 4-week cycle on days 1, 8, 15, and 22 for the first 3 months. From cycles 4 to 12, it is given on days 1 and 15. Lenalidomide is given at 25 mg/d from days 1 to 21 in a cycle of 28 days. If they have stable disease [SD] at the end of 12 cycles, they receive tafasitamab alone as maintenance every 2 weeks until progression of disease.7
The primary end point was ORR. There were several secondary end points including safety, PFS, [and] DOR. The primary end point analysis data cutoff was November 2018. The minimum follow-up was 12 months and median follow-up was 17.3 months.7
The median age of patients was 72 [years]. This was not a randomized study, but a single-arm study. Patients with an IPI score from 0 to 2 were 49% and [51% had a high IPI of 3 to 5].7,8 Patients with Ann Arbor stage I to II disease made up 25% of the patients and 75% were in the higher stage of III to IV. Elevated LDH was seen in 56% of patients and it was not elevated in 44%.
The median previous lines of therapy was 2, ranging from 1 to 4 with 50% of patients having had 1 line of therapy, 43% having 2 lines, 6% having 3 lines, and 1% having 4. Primary refractory disease was seen in 19% of patients and those refractory to the last prior line of therapy were 44%. Prior SCT was seen in 11% of patients, but most of the patients had not had a transplant or were transplant ineligible. For cell of origin, 10% were GCB, 25% non-GCB, and 65% other.
The best response at 35 months was a CR rate of 40%, PR rate of 17.5%, SD [16.3%], and progression was seen in 16%, and 10% were not evaluable.7,8 The ORR was 57.5% with a median DOR of 43.9 months. The median time to response was 2.1 months. The median PFS was 11.6 months. Certainly, patients with CRs had to have better PFS and OS. The OS rate at a median of 42 months was 33.5 months, 12-month OS was 74%, and 18-month OS rate was 64%.8 That is pretty good for patients who at least achieve a CR, but there is toxicity.
I know it is not easy to give lenalidomide. Neutropenia, anemia, and thrombocytopenia were seen in more than 20% of patients. Grade 3 and 4 neutropenia were seen in 27% and 21% of patients, respectively. Febrile neutropenia was not high; it was about [12%]. Lymphopenia was noted, but agranulocytosis was [only seen in 1 patient]. Some other AEs were fatigue, diarrhea, cough, fever, peripheral edema, urinary tract infections, and decreased appetite. Most were grades 1 and 2, fewer were grade 3, and almost none were grade 4.5,7
Serious AEs were noted in about 51% of patients [which is] significant, but serious AEs suspected to be treatment related were observed in 19%. Discontinuation of the combination due to AEs was seen in about 12% of patients. AEs of special interest were present in 9% and those leading to death were about 13%. Discontinuation of lenalidomide at the end of 12 cycles or due to toxicity reduced the incidence and severity of AEs, so toxicity was mostly due to lenalidomide. The dose of lenalidomide had to be reduced in about 45% of patients. Most patients [77.5%] were able to tolerate about 20 mg/d of lenalidomide over the duration of treatment of 1 year.5,7
Again, there are fewer AEs with tafasitamab therapy alone and most of the AEs happen in combination with lenalidomide, but there was a lot of hematologic toxicity. With the CD19 therapy, you do not want to interfere with potential CAR T-cell therapy, which is also CD19 directed. There are certain limitations to this combination.
What data support the use of loncastuximab tesirine in relapsed or refractory DLBCL?
Loncastuximab tesirine is a novel CD19-based ADC combined with PBD [pyrrolobenzodiazepine]. PBD is a cytotoxic DNA minor groove cross-linking payload that seemed to have a good preclinical therapeutic index.9 Loncastuximab was studied in relapsed/refractory DLBCL as a single agent leading to its accelerated approval by the FDA in April 2021 for DLBCL after more than 2 lines of therapy, high-grade BCL [HGBCL], transformed DLBCL, and DLBCL not otherwise specified [NOS].10
Loncastuximab was studied in the phase 2 LOTIS-2 study [NCT03589469] for relapsed or refractory DLBCL. Eligible patients had to have failed 2 or more lines of systemic therapy. They had to show CD19 positivity on biopsy if they had received prior CD19 therapy such as CAR T-cell therapy. They had good ECOG performance status of 0 to 2. They could have had a prior autologous or allogeneic SCT provided the toxicities had resolved. Loncastuximab is a 30-minute infusion every 3 weeks for up to 1 year. The first 2 cycles were at 0.15 mg/kg and afterward, the dose was reduced to 0.075 mg/kg. The follow-up was up to 3 years.11
The primary end point was ORR. The secondary end points were DOR, CR, PFS, OS, safety, quality of life, etc. The primary antitumor activity and safety analysis was done in the as-treated population—those who had received at least 1 dose of loncastuximab—when all responding patients had more than 6 months of follow-up after the initial documented response.11
There were 145 patients; 41% were female and 59% were male. The median age was 66 [years]. An important point is that they included patients with DLBCL, HGBCL, and primary mediastinal BCL [PMBCL]. Out of these, 10% were double hit or triple hit, 14% were double or triple expressors, and 20% had transformed disease. So, patients who were included had high-risk histology as well.11
Patients with early-stage disease [comprised] 23% and [those with] stage III and [stage] IV [disease comprised] 77%. GCB subtype present in 33%, 16% were non-GCB, and others were 51%. The median number of prior therapies was 3 with [approximately] 43% of patients having had 2 lines of therapy, 24% had 3, and 32% of patients had more than 3. For first-line systemic therapy response, 68% had relapsed disease, and 20% had refractory disease.
Patients refractory to the last line of therapy [comprised] 58% and those refractory to all prior lines of therapy [comprised] 79%. These were patients who were advanced. Two patients had prior allogeneic SCT, 21 had prior autologous SCT, and 13 patients, which is 9%, had prior CAR T-cell therapy.11 Out of 145 patients, 24% had a CR and 24% a PR. The ORR was 48%. The median time to first response was 41 days. Out of the 35 patients who had CRs, 57% were maintained at the data cutoff point. Most responses were after 2 cycles, so about 6 weeks. The mean number of treatment cycles received was 4.5. The responses were reported from the March 1, 2021, data cutoff.11
The median DOR by the April 6, 2020, data cutoff was 10.3 months and by the following year using the March 1, 2021, data cutoff it was 13.4 months.11,12 The median DOR for patients with a CR was 13.4 months and was not reached a year later. The patients with a CR seemed to have a longer DOR.
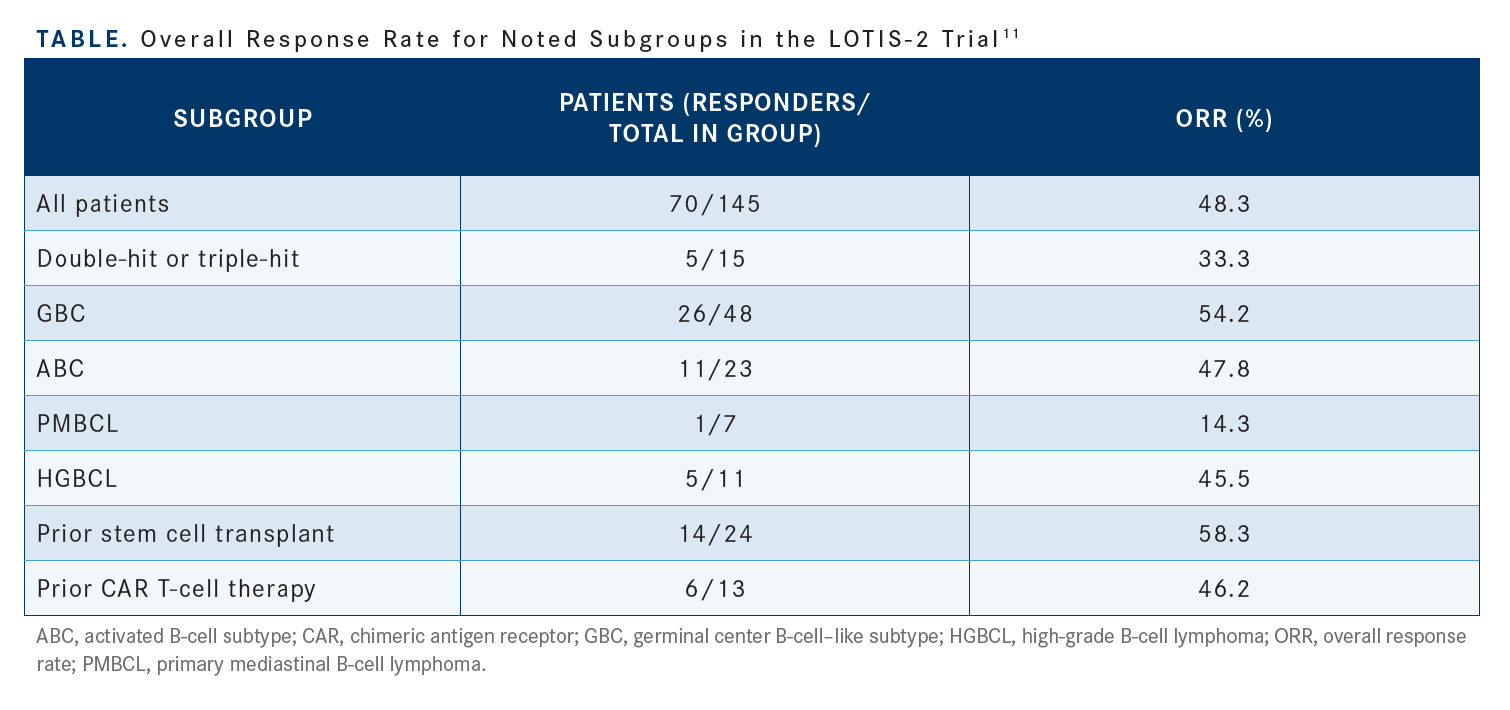
The subgroup analysis [explored] patients with HGBCL, prior SCT, and prior CAR T-cell therapy and the data seemed to favor the use of loncastuximab in these patient subgroups [Table11]. The cell of origin and transformed disease did not seem to make a difference. Again, this is an agent that can be used in patients with HGBCL or patients who have had prior CAR T-cell therapy if they continue to express CD19.11
For subsequent treatment for patients who progressed on loncastuximab, 15 patients received CD19-directed CAR T-cell therapy with a response rate of 46.7%. If they continue to express CD19, they don’t lose their response after CAR T-cell therapy. Nine patients proceeded to SCT as consolidation after responding to loncastuximab.11
If you are going to use loncastuximab before CAR T-cell therapy, you have got to be aware that it may affect the CD19 expression, but it seems like patients have received CD19-directed CAR T-cell therapy after loncastuximab.
Loncastuximab was active even in patients who had some challenging subgroups like HGBCL where the CR rate was 45.5%, and for DLBCL-NOS it was 50.4%.
The time to first CR in HGBCL was about 79 days and it is 43 days for DLBCL-NOS. The time to first response was 43 days in HGBCL and 41 days in DLBCL-NOS. The best response to CAR T-cell therapy after loncastuximab in 13 patients was a CR of 54% and PR of 15%. The best response to loncastuximab after CAR T-cell therapy relapse was a CR rate of 15.4% and PR rate of 30.8%.13 Again, this shows you that you can probably combine these therapies provided the patients continue to have CD19 expression. Again, where to place them is still obviously not clear and that is why the NCCN [National Comprehensive Cancer Network] guidelines just list them. It is left to the investigator or the treating physician to decide.
The rate of AEs under the age of 65 [years] and over the age of 65 [years] were similar. [The overall rate of AEs was] 100%; everybody had some AEs. GGT [γ-Glutamyltransferase] increase was an issue with loncastuximab. It was 50% in those younger than [age 65 years] and it was about 33% in those over [age 65 years]. Neutropenia was seen in 52.3% of patients under age 65 years and 30% of those over [age 65 years]. Thrombocytopenia was seen in 43.1% of patients under age 65 years and 25% of those over age 65 years. There was also fatigue, anemia, and nausea. Peripheral edema was common as well at 16.9% in those under age 65 years and 22.5% in those over age 65 years.11 However, the etiology of this is not quite clear.
The rise of GGT did lead to some discontinuations in this trial. The most common AEs were neutropenia, thrombocytopenia, GGT increase, and anemia. Treatment-related AEs that led to treatment discontinuation were seen in 17.9% of patients. The most common causes of discontinuations were GGT increase, peripheral edema, and localized edema. They used spironolactone to treat peripheral edema.11
It seems like patients over [age 65 years] tolerated the treatment reasonably well. Oral dexamethasone was used and the patients had to be counseled to avoid sun exposure because of the skin toxicity associated with sun exposure. There were some treatment delays permitted in the study to manage some of these toxicities. Again, there was judicious use of spironolactone to counteract the peripheral edema if a patient gained more than 1 kg.11
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphoma, version 5.2022. Accessed August 17, 2022. https://bit.ly/3dCLi1u
2. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
3. FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. FDA. June 10, 2019. Accessed August 17, 2022. https://bit.ly/2XEazLP
4. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi:10.1182/ bloodadvances.2021005794
5. Monjuvi (tafasitamab). Prescribing information. MorphoSys US Inc; 2020. Accessed August 30, 2022. https://bit.ly/2S38ZW7
6. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. August 3, 2020. Accessed August 17, 2022. https://bit. ly/3bodb9v
7. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
8. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. doi:10.3324/haematol.2020.275958
9. Zammarchi F, Corbett S, Adams L, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131(10):1094-1105. doi:10.1182/blood-2017-10-813493
10. FDA grants accelerated approval to loncastuximab tesirine-lpyl for large B-cell lymphoma. FDA. April 23, 2021. Accessed August 17, 2022. https:// bit.ly/3ApwfBc
11. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:10.1016/ S1470-2045(21)00139-X
12. Caimi P, Ai WZ, Alderuccio JP, et al. Duration of response to loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma by demographic and clinical characteristics: subgroup analyses from LOTIS 2. J Clin Oncol. 2021;39(suppl 15):7546. doi:10.1200/JCO.2021.39.15_suppl.7546
13. Caimi PF, Ardeshna KM, Reid E, et al. The antiCD19 antibody drug immunoconjugate loncastuximab achieves responses in DLBCL relapsing after antiCD19 CAR-T cell therapy. Clin Lymphoma Myeloma Leuk. 2022;22(5):e335- e339. doi:10.1016/j.clml.2021.11.005

Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen