Roundtable Discussion: Examining Treatment Options After Adjuvant Chemotherapy for TNBC
During a case-based roundtable event, Christos Vaklavas, MD moderated a discussion about treatment option for a 48-year-old woman with triple negative breast cancer following adjuvant chemotherapy.
Christos Vaklavas, MD


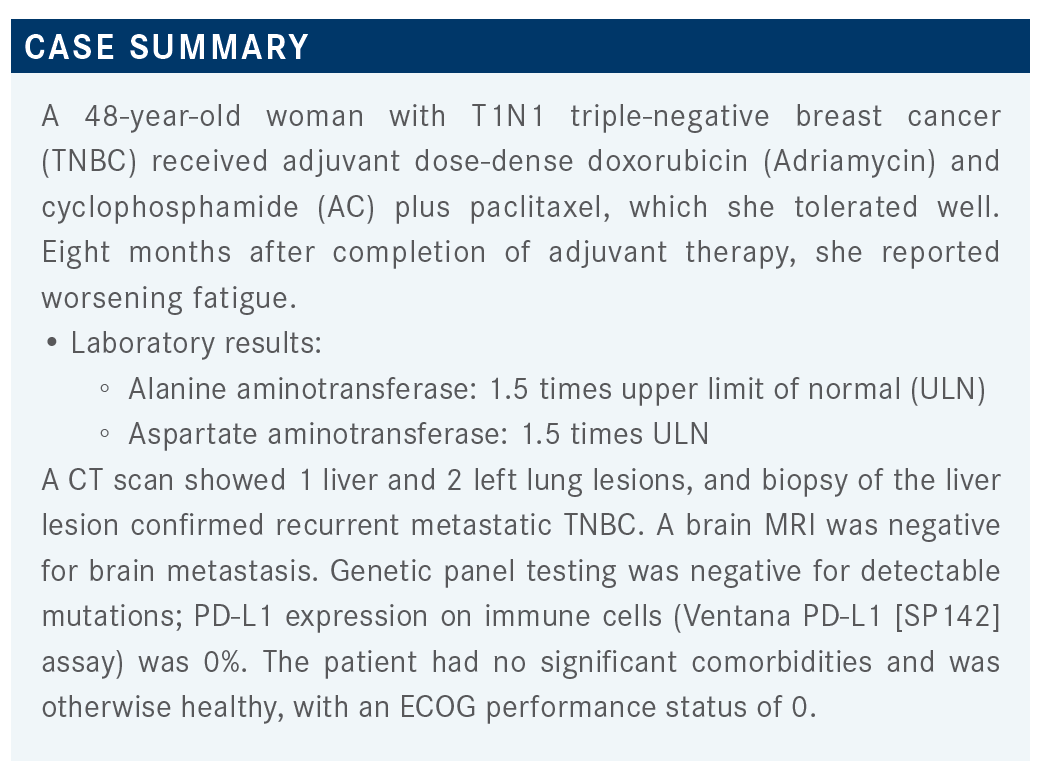
VAKLAVAS: I’m curious to see what intravenous single-agent chemotherapy some of you would give. For those who would give oral single-agent chemotherapy, I think this would be capecitabine [Xeloda]. For combination chemotherapy, it’s an early recurrence, so carboplatin and gemcitabine may be a very good option for her. And 43% of participants would do a clinical trial. That is a very interesting result.
I’d love to see what clinical trial you would enroll her in. If you chose chemotherapy or other, what agent or regimen are you most likely going to recommend?
LEE: I would give her carboplatin and gemcitabine.
VAKLAVAS: These are 2 agents that the patient has not seen, and it makes sense. How would others treat this patient?
SRIVASTAVA: I would agree with carboplatin, especially if LFTs [liver function tests] are elevated also. Carboplatin and gemcitabine.
VAKLAVAS: Who would choose oral single-agent chemotherapy, [namely] capecitabine?
NALLAPAREDDY: Yes, I would try capecitabine because there are only 1 or 2 liver lesions and not too much disease [volume].
VAKLAVAS: [Yes, there is] not too much disease. They’re going to burn out with carboplatin and gemcitabine.
RANA: I selected capecitabine as well. Disease burden is not very high; she can have a good quality of life with oral chemotherapy.
VAKLAVAS: Well, back in the days when the standard of care was a little bit different, there was a clinical study [whose results] showed that combination chemotherapy may give patients a more rapid response, but it may not necessarily give them great mileage in terms of overall survival.1 [However,] that study was done decades ago. Of course, with the current standard of care having changed, we don’t know how [the findings from] those studies are applicable today. There’s no right answer for this one. The National Comprehensive Cancer Network [NCCN] guidelines leave it wide open.2
Nearly half of you voted for a clinical trial. I’m eager to find out what clinical trial you are going to enroll her in. What kind of clinical trial do you have in your institution? There is a phase 3 clinical trial with datopotamab deruxtecan [Dato-DXd] going on right now and they’re actively enrolling [TROPION-Breast02; NCT05374512]. It’s for PD-L1–negative patients with metastatic or unresectable TNBC.
This is the antibody-drug conjugate from AstraZeneca and Daiichi Sankyo. It’s a phase 3 clinical study that compares Dato-DXd against treatment of physician’s choice, and I don’t [specifically] remember the treatments of physician choice but I’m sure it’s going to be paclitaxel, nab-paclitaxel [Abraxane], gemcitabine, carboplatin—the standard chemotherapies that we’re using for TNBC.
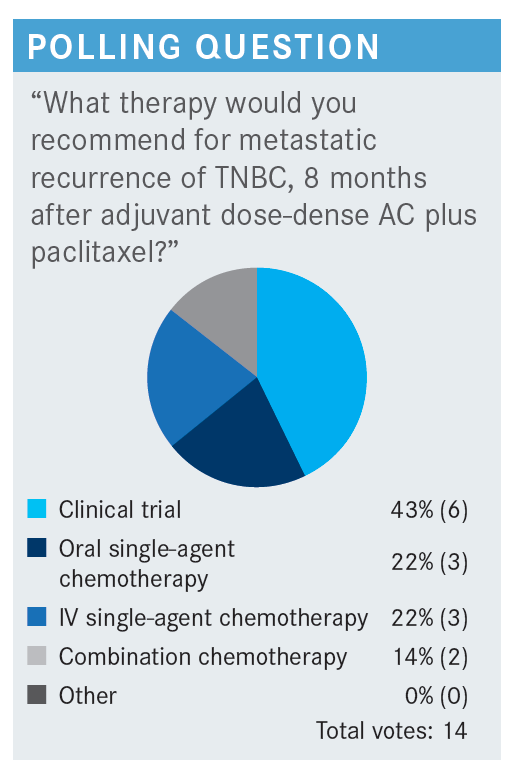
VAKLAVAS: Would your frontline approach differ if the relapse occurred 24 months after her adjuvant chemotherapy? This would be considered not necessarily a late relapse, but at least a nonearly relapse. Would you rechallenge her with paclitaxel or nab-paclitaxel? Those of you that voted for IV single-agent chemotherapy, I’m curious to find out what chemotherapy you’re going to give.
FAHED: Yes, I changed my answer from combination to single agent. Mainly it was [because of] the idea that I will rechallenge with a taxane, most likely nab-paclitaxel.
HAO: I will also rechallenge with paclitaxel. It’s after 24 months, long-time remission, and low disease burden. If the patient had good tolerance to paclitaxel, I would try it again.
VAKLAVAS: Great thoughts. How about capecitabine?
SRIVASTAVA: I’d be comfortable giving capecitabine. The only thing that is interesting is even though she has 1 liver lesion, LFTs are high in this case, which makes you wonder if she has some diffuse disease or something that is not logically visible. But for the sake of the question, in early relapse I would be more worried that within 8 months she’s having so much disease come back. But now, 2 years later, I think any of those options—either retrying paclitaxel or single-agent capecitabine—[could be used, but] I’d be a little concerned [as to] why her LFTs are high if it’s just a single liver lesion.
VAKLAVAS: All chemotherapies probably, more or less, can give you very similar mileage. I like capecitabine because it’s an oral agent and it’s easy to tweak. [With] most chemotherapies, once you inject them there’s no way to pull them out; that’s it. So the first therapy is usually the best therapy; they can get a pretty good progression-free survival [PFS] out of it.
I would like to hear [from] the people who chose combination chemotherapy. How can you defend yourselves? Do you want to give carboplatin and gemcitabine?
LEE: Yes, I want to give a combination because I have single-agent capecitabine later. I’m worried about chemotherapy attrition in this patient. I want to shrink it down as quickly as possible and then consider maintenance.
VAKLAVAS: Yes, that’s fair enough. [This would be similar to a] leukemia regimen: induction followed by maintenance.
MUSHTAQ: My thought process is a combination, either carboplatin and gemcitabine or gemcitabine and paclitaxel. Shrink the tumor, probably with carboplatin, drop it after 3 or 4 cycles, and keep gemcitabine as a single agent.
VAKLAVAS: Are there other agents that you’re going to use as single-agent chemotherapy?
GEORGE: I would have still done the carboplatin and gemcitabine, but doxorubicin liposome injection [Doxil] could be very reasonable too, depending on her EF [ejection fraction].
SHINDE: Was BRCA tested for her? If that could be done, and if she’s BRCA positive, olaparib [Lynparza] might also be an option.
VAKLAVAS: Well, she’s BRCA negative, [because the genetic panel testing was negative], but this is a very good comment. Whenever you deal with newly diagnosed metastatic TNBC, if you read the NCCN guidelines, they recommend that you run next-generation sequencing.2 You can run whichever test you want, but you don’t want to miss PD-L1 positivity, you don’t want to miss BRCA mutations, and you don’t want to miss the PALB2 mutations.
The PALB2 mutations behave in the same way as the BRCA1 and the BRCA2 mutations. PALB2 essentially means “partner and localizer of BRCA2 gene,” so it’s essentially the same as the BRCA2 mutation. You don’t want to miss a high tumor mutational burden [TMB] because you have those special treatment considerations with the PARP inhibitors and immunotherapies, where the patients can actually have long-term remissions with very reasonable toxicities.
The NCCN guidelines, if you read them carefully, recommend next-generation sequencing even for patients with HER2-positive disease, where the treatment paradigm is very well outlined.2 But it’s very rare. I haven’t seen it in my career—[a patient] having a BRCA1 or potentially a BRCA2 mutation and [also] having a HER2-amplified or HER2-positive tumor.

VAKLAVAS: Thinking about your therapeutic options for this patient with this rapid recurrence—we’re skipping back to the 8-month relapse—having progressed after 6 months of gemcitabine and carboplatin...which agent are you going to consider as a second line in the metastatic setting?
What is your ideal sequence in this patient with metastatic PD-L1–negative and BRCA-negative TNBC? This patient has seen 5 different chemotherapies: dose-dense doxorubicin, cyclophosphamide, paclitaxel; 8 months later, you’re treating her with carboplatin and gemcitabine. She also had a PR for only 6 months.
ALLURI: I have this exact patient. I moved on to sacituzumab govitecan [Trodelvy] after those 5 cytotoxic agents and I think she had at least 8 months of response, and she has just about started to progress again [From the Data3]. I’m looking for clinical trials, and right now she’s on eribulin [Halaven], but I think soon I’m going to run out of options.
RANA: Sacituzumab govitecan sounds like the most reasonable option. Even if we were to try capecitabine or eribulin, the [patient’s] response to them would last a few months. I think sacituzumab govitecan should be the way to go.

VAKLAVAS: What was [sacituzumab govitecan’s] efficacy, and how about its tolerability? How do you counsel your patients regarding sacituzumab govitecan’s toxicity and mechanism of action?
ONWERE: I have a single patient on therapy. She was actually estrogen receptor/progesterone receptor [ER/PR] positive, and now she’s ER/PR negative. She’s been on therapy now for about 3 to 4 months and doing well. The main issue is the usual alopecia, but she seems to be having an excellent quality of life.
SRIVASTAVA: My experience was not that good. I used it in the fourth or fifth line in a patient who started off as HER2 positive then changed [to TNBC].
VAKLAVAS: I think that this disease is a little bit different and tends to behave more indolently compared with up-front TNBC, especially some variants like metaplastic TNBC that are aggressive. Those converters that tend to respond have a little bit longer PFS with each line of therapy.
SRIVASTAVA: That patient actually ended up with ileitis. She didn’t do well, then went on to hospice, [after] 2 years [receiving multiple lines of therapy]. Another patient had cytopenias. Outside of that, I haven’t used it too much in breast cancer.
VAKLAVAS: I think the FDA thought about the limited treatment options in aggressive metastatic TNBC; that’s why they upheld the 12-month interval between completion of treatment in the adjuvant and neoadjuvant setting and metastatic setting. That’s probably why they decided to allow it to be used in patients who have relapsed disease, as a second-line therapy.

VAKLAVAS: What would you use as a next-line therapy for patients who progress on sacituzumab govitecan? Would you use eribulin on the basis of the [data from the] clinical studies?
ARANGO: That’s what I would answer after sacituzumab govitecan: eribulin.
VAKLAVAS: [Would anyone use] any other chemotherapy? How about cisplatin or carboplatin if you have not used them [before]? If the patient has a germline BRCA1/BRCA2 mutation, has seen olaparib, and has seen sacituzumab govitecan, would you use carboplatin or cisplatin? We now know, from the [findings from the] TBCRC [Translational Breast Cancer Research Consortium] studies, that carboplatin or cisplatin may not be superior to paclitaxel, at least in patients with early-stage breast cancer.4
ALLURI: I think one of the challenges now is going to be with the KEYNOTE-522 [NCT03036488] regimen, where they get this cocktail of carboplatin, anthracycline, taxane, and immunotherapy.5 If they still relapse…what do you do in that situation? Because that is getting increasingly common, where you use up most of your potent agents up front.
VAKLAVAS: What factors will influence your decision about the next-line therapy? [These include] safety and tolerability, performance status, age, symptom burden, cumulative toxicities, comorbidities, duration of response to prior therapy, and change to a novel class of therapy. Are there other considerations in picking…the next-line therapy after progression on sacituzumab govitecan?
ALLURI: I did have a patient who pretty much progressed on everything, was PD-L1–negative, but had a high TMB. I had to go to pembrolizumab [Keytruda], although the patient didn’t make it [so I did not] see if she responded. She had to go into hospice pretty quickly.
VAKLAVAS: I’m afraid that’s the reality. If you look at those [Kaplan-Meier] PFS curves, there’s a steep decline in the beginning, and then later on there is a flat line. Your patient was probably on the steep descent.
REFERENCES:
1. Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol. 2003;21(4):588-592. doi:10.1200/JCO.2003.08.013
2.NCCN. Clinical Practice Guidelines in Oncology. Breast cancer, version 4.2022. Accessed September 7, 2022. https://bit.ly/3kxcYWm
3. Bardia A, Hurvitz SA, Tolaney SM, et al; ASCENT Clinical Trial Investigators. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541. doi:10.1056/NEJMoa2028485
4. Mayer EL, Abramson V, Jankowitz R, et al. TBCRC 030: a phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: evaluating the homologous recombination deficiency (HRD) biomarker. Ann Oncol. 2020;31(11):1518-1525. doi:10.1016/j.annonc.2020.08.2064
5. Schmid P, Cortes J, Pusztai L, et al; KEYNOTE-522 Investigators.Pembrolizumab forearly triple-negative breast cancer.N Engl J Med. 2020;382(9):810-821. doi:10.1056/NEJMoa1910549
