Pothuri Reviews First-line PARP Inhibition in Advanced Ovarian Cancer
During a case-based roundtable event, Bhavana Pothuri, MD, discussed the use of PARP inhibitors to treat first-line advanced ovarian cancer.
Bhavana Pothuri, MD

Targeted OncologyTM: What is first-line maintenance therapy for advanced ovarian cancer?
POTHURI: It is treatment given to patients who’ve achieved a remission after their initial treatment, which is surgery and induction chemotherapy, with the aim of prolonging the duration of remission. There are 2 types of maintenance therapy. Continuation maintenance is continuation of 1 or more of the drugs that was used in the induction regimen. Bevacizumab [Avastin] is a continuation maintenance therapy. Switching maintenance is introducing a new agent after completion of the induction treatment, and an example of that is using PARP inhibitors.1
Why is maintenance therapy important in this setting?
A compilation of the control arms in large phase 3 trials in ovarian cancer show that overall, the median progression-free survival [PFS] after chemotherapy in the control arm is between 8 and 12 months.2-5 We’ll keep the SOLO-1 trial [NCT01844986] out of the [median] because it only included BRCA mutation carriers. But clearly, there is a need for improvement in median PFS.
I’m pleased to say that from 2014 to 2020, we’ve had a flurry of FDA approvals in ovarian cancer with 14 newly approved drugs. That’s more approvals in the past 6 years than the prior 60 years combined, so it’s an exciting time to be in this field. Most of these are PARP inhibitors, 9 of those 14 approvals. We’ll focus on PARP inhibitors that have been approved in the frontline setting.
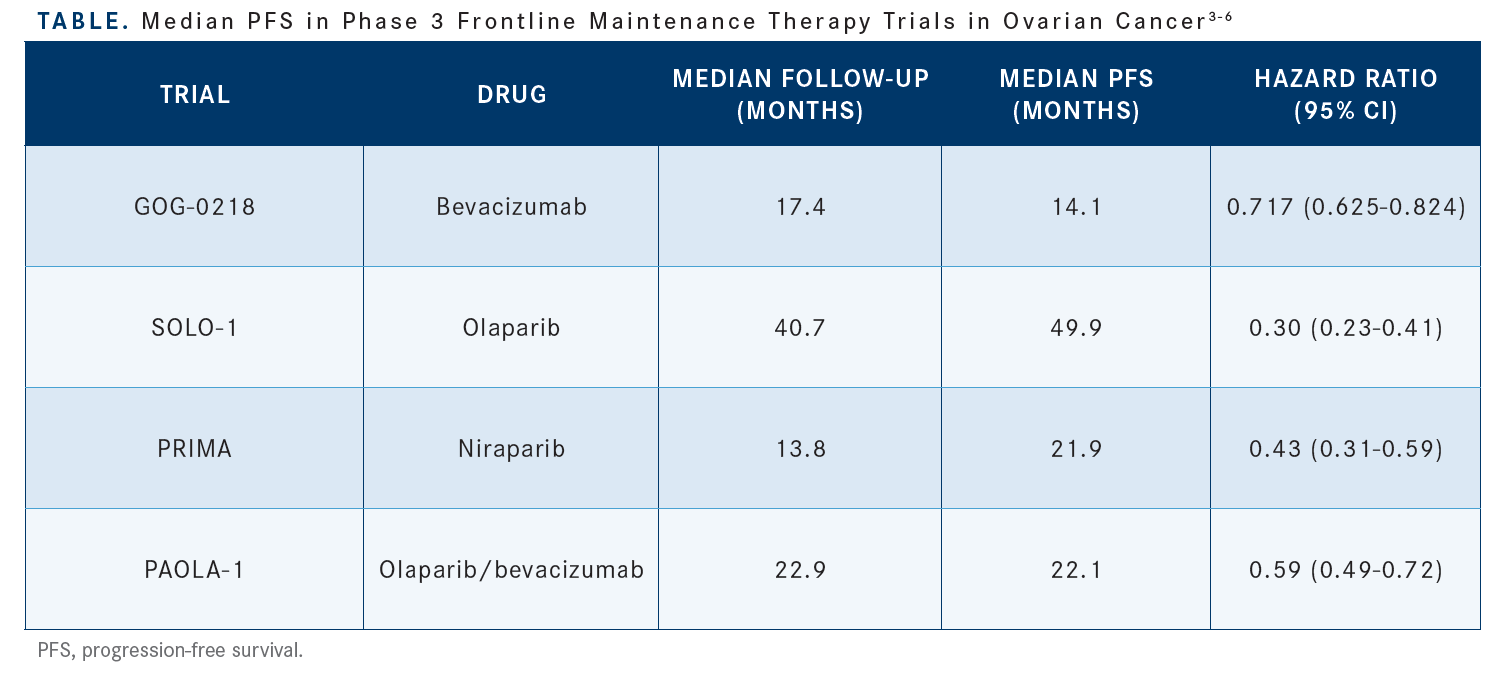
There are phase 3 trials for first-line maintenance therapy after platinum-based therapy. We’ll go through 3 trials with the PARP inhibitors in detail. But it’s also important to keep in mind that there is a role for bevacizumab. GOG-0218 [NCT00262847] looked at bevacizumab, as did ICON7 [NCT00483782]. The maintenance was for up to 22 cycles after chemotherapy. The SOLO-1 trial looked only at BRCA mutation carriers on maintenance therapy with olaparib [Lynparza]. The PRIMA trial [NCT02655016] looked at niraparib [Zejula] in all-comers, and PAOLA-1 [NCT02477644] looked at the combination of bevacizumab and olaparib in all-comers.3-6
The [NCCN (National Comprehensive Cancer Network) guidelines show where] we are in terms of our treatment.7 I hope to simplify all of them for you by walking you through each of the recommendations.
What data support the use of olaparib maintenance after first-line chemotherapy?
The SOLO-1 trial looked at olaparib maintenance after first-line chemotherapy in only BRCA1/2 carriers who were newly diagnosed and had received prior platinum-based chemotherapy. They were randomized 2:1, olaparib 300 mg twice a day to placebo. The primary end point was investigator-assessed PFS. Secondary end points were PFS, OS [overall survival], and time to subsequent therapy. In the trial, 391 patients had germline BRCA1/2, 2 had somatic BRCA, [and 1 had BRCA with variant of unknown significance]. Most of these patients had NED [no evidence of disease] and good performance status.4
[The median PFS after 5 years of follow-up was 56.0 months for olaparib vs 13.8 months for placebo] with a hazard ratio of 0.33 [95% CI, 0.25-0.43], which is highly significant. The most important aspect is that in this trial they ended their treatment at 2 years, but even 3 years later 48% of patients remained free of disease with olaparib vs 21% [with] the placebo, so clearly an impressive benefit.8 Again, this is a situation where we’re curing some of these patients, and that is why it is the standard of care in a BRCA carrier to receive a PARP inhibitor post chemotherapy.
The subgroup analysis at 41 months of follow-up showed all subsets had a clear benefit with olaparib.4
The most common adverse events [AEs] were nausea, fatigue, vomiting, anemia, diarrhea, and constipation. Most of these are manageable, and in the original report there wasn’t a greater risk of AML [acute myeloid leukemia] and MDS [myelodysplastic syndrome].4,8
What data support the use of niraparib in high-risk patients after first-line chemotherapy?
The PRIMA/ENGOT-OV26/GOG-3012 trial had niraparib vs placebo in a much sicker population. Two-thirds of these patients were receiving neoadjuvant chemotherapy, and one-third of them had stage IV disease. They should have had a CR [complete response] or PR [partial response] after frontline platinum-based chemotherapy. Keep in mind that even though they were a high-risk group of patients, they all responded well to platinum therapy. They were then randomized 2:1, niraparib 300 mg vs placebo, and the primary end point was PFS by BICR [blinded independent central review]. This was a hierarchical analysis, first in the HRD [homologous recombination deficiency] group and then in the all-comers population. They were stratified by neoadjuvant chemotherapy, tumor response— CR vs PR—after chemotherapy, and HRD status—positive, negative, or undetermined.5
This was a much sicker population with more advanced disease. The percentage of those on the fixed dose who had neoadjuvant chemotherapy was quite high at 66.7%. Patients were on either fixed 300-mg dosing or the individualized dosing. About two-thirds of the way through the trial, the trial was amended and patients who were less than 77 kg or had a platelet count of less than 150,000/μL were started at 200 mg as opposed to 300 mg.
For the efficacy data, the first analysis was done in the HRD population. The PFS hazard ratio was 0.43 [95% CI, 0.31-0.59; P < .001] and the median PFS benefit was almost double at [21.9 months for niraparib vs 10.4 months for placebo]. Because these data were positive, the overall population was looked at and the PFS hazard ratio was still quite good at 0.62 [95% CI, 0.50-0.76; P < .001]. The median PFS, while not quite as impressive as the HRD population, still had a benefit at 13.8 months vs 8.2 months.
When they looked at the HRD BRCA mutant vs the HRD BRCA wild type, the greatest benefit was in the BRCA-mutated population, with a PFS hazard ratio of 0.40 [95% CI, 0.27-0.62]. But the HRD subset that was BRCA wild-type also had a significant improvement, with a PFS hazard ratio of 0.50 [95% CI, 0.31-0.83]. Niraparib had a benefit across all subgroups.
Of course, safety is important. The main all-grade AEs were anemia, nausea, thrombocytopenia, constipation, and fatigue. So, the individualized dosing was initiated.5 At the [2022 annual meeting for the SGO (Society for Gynecologic Oncology)], they presented the PRIME data that used the individualized [findings]. But, overall, there were no new safety signals, and the toxicities were representative of this class of agents.
What data support the use of bevacizumab plus olaparib as first-line maintenance therapy?
The PAOLA-1 trial looked at the combination of bevacizumab with olaparib as maintenance therapy. This was in patients with newly diagnosed advanced ovarian cancer who completed their surgery and platinum-based chemotherapy, and again had a PR or CR with at least 4 cycles of chemotherapy. They allowed neoadjuvant chemotherapy, and patients were randomized either to receive bevacizumab or olaparib and bevacizumab.6
The olaparib plus bevacizumab outperformed the placebo plus bevacizumab arm. The hazard ratio [for disease progression or death] was 0.59 [95% CI, 0.49-0.72; P < .001], again noting a benefit for the combination.
In the prespecified analyses, the PFS in the HRD-positive subgroup that included the tBRCA [tumor BRCA]-mutated patients saw the most impressive benefit with a hazard ratio of 0.33 [95% CI, 0.25-0.45]. The median PFS was 37.2 months for the combination vs 17.7 months for the placebo arm, so that’s quite significant. The HRD-positive subgroup that didn’t include the tBRCA mutations still had a significant hazard ratio of 0.43 [95% CI, 0.28-0.66]. The hazard ratio in the HRD-negative and unknown subgroup was 0.92 [95% CI, 0.72-1.17].6 For this reason, the FDA gave approval in the HRD-positive subgroup. Because the study was not powered to look at this specifically, we were a little surprised that the approval came in this subgroup. But, nonetheless, we have it.
The most common any-grade AEs were fatigue and nausea. Hypertension is also an AE related to this regimen given the combination of bevacizumab, and hypertension was also noted in niraparib, so it’s important to keep in mind. There was about a 20% dose discontinuation rate, so keep that in mind with the combination.6 The rate is much lower when you’re using a single-agent PARP inhibitor.
It’s important to utilize frontline maintenance therapy in ovarian cancer…. Across studies there was a benefit to maintenance therapy, whether it was bevacizumab in GOG-0218 or the PARP inhibitors or the combination of bevacizumab and olaparib in PAOLA-1 [Table3-6].
What is the standard systemic treatment for newly diagnosed advanced epithelial ovarian cancer in 2022?
I’ve simplified my thinking about this, and there are 3 main decisions we must make. The first is do we give neoadjuvant chemotherapy or do primary debulking. It is important that all patients are referred to gynecologic oncologists to determine the right decision. Then the patients can undergo their chemotherapy.
The second decision is do you use bevacizumab or not. If you use bevacizumab during chemotherapy, whether it be in the neoadjuvant setting or post chemotherapy, then you can decide based on their BRCA mutation and HRD status. If they’re HRP [homologous recombination proficient], I typically continue the bevacizumab and that’s because most of the data from the GOG-0218 and ICON7 [trials] showed that the benefit of bevacizumab was when it was continued in the maintenance setting.
[The third decision is whether to add a PARP inhibitor]. So, if they are HRD positive, I add olaparib to that. Now, if they didn’t get bevacizumab in their treatment, you can either give a patient niraparib regardless of BRCA or HRD status. If they are BRCA mutant, you can also give them olaparib. If they are HRD-positive, you can add bevacizumab.
What I tend to do is, if I haven’t started the bevacizumab, I don’t start it with olaparib. I usually continue with either if they’re a BRCA-mutant carrier, and I consider olaparib or give them niraparib if they’re not. There are patients to whom you may consider not giving a PARP inhibitor and just observing, but the American Society of Clinical Oncology guidelines suggest that we offer it to all patients.3-6
Anemia, thrombocytopenia, and neutropenia are all noted AEs with these therapies. Nausea, vomiting, and fatigue, too. Grade 3 or 4 hypertension in the PRIMA trial of niraparib was about 5% and then with olaparib plus bevacizumab, it’s about 19%. The thrombocytopenia risk in niraparib was about 39%. I think once we’ve figured out how to use it, that once-a-day dosing does make a difference for patients.9,10
What is the individualized niraparib dosing based on baseline body weight and platelet count? What are the dose adjustments for adverse reactions to niraparib and olaparib?
If the patient’s baseline body weight is less than 77 kg or their platelets are less than 150,000/μL, then you should start at 200 mg once daily. Otherwise, it is 300 mg. This led to an approximate 50% reduction in the thrombocytopenia risk and improved tolerability.10 For PARP inhibitors, the hematologic toxicity is important. I give patients at least 8 weeks to recover from their primary chemotherapy, so between 8 and 12 weeks is a good time to start PARP maintenance therapy.
The starting dose of olaparib is 300 mg twice a day. The first dose reduction is to 250 mg twice a day, and then the second dose reduction is 200 mg twice a day. With niraparib, it’s 300 mg if the body weight is greater than 77 kg and platelets are greater than 150,000/μL and then the first dose reduction is to 200 mg and then the second is 100 mg. If the weight is 77 kg or lower or platelets are less than 150,000/μL, you start at 200 mg and 100 mg is the first dose reduction and then you stop.9,10
A Chinese study on niraparib [called PRIME (NCT03705156)] was presented at the annual 2022 SGO meeting.11 It was a randomized phase 3 placebo-controlled trial with the niraparib individualized starting dose. It’s a 2:1 randomization of niraparib vs placebo and they continued it for 3 years or until progression. The primary end point was PFS by blinded independent central review. They started with 200 mg if their weights were less than 77 kg, and the platelet count was less than 150,000/μL.
There was still efficacy with a PFS hazard ratio of 0.45 [95% CI, 0.34-0.60; P < .001]. The treatment was tolerable. I think the important thing to note in this study was that the treatment discontinuation rate was low at 6.7% [vs 5.4% in the placebo group].11 If I recall, most patients were started at the lower dose. Nonetheless, it does give us some confidence that this dose is efficacious. This is a prospective evaluation of the individualized starting dose since in the PRIMA trial it was adopted two-thirds of the way in.
REFERENCES:
1. Gentzler RD, Patel JD. Maintenance treatment after induction therapy in non-small cell lung cancer: latest evidence and clinical implications.Ther Adv Med Oncol. 2014;6(1):4-15. doi:10.1177/1758834013510589
2. Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Group and the NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer.N Engl J Med. 2010;363(10):943-953. doi:10.1056/NEJMoa0908806
3. Burger RA, Brady MF, Bookman MA, et al; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer.N Engl J Med. 2011;365(26):2473-2483. doi:10.1056/NEJMoa1104390
4. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer.N Engl J Med. 2018;379(26):2495-2505. doi:10.1056/NEJMoa1810858
5. González-Martín A, Pothuri B, Vergote I, et al;PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer.N EnglJ Med. 2019;381(25):2391-2402. doi:10.1056/NEJMoa1910962
6. Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416-2428. doi:10.1056/NEJMoa1911361
7. NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2022. Accessed August 30,2022. https://bit.ly/35ZX9PA
8. Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 .Lancet Oncol. 2021;22(12):1721-1731. doi:10.1016/S1470-2045(21)00531-3
9. Lynparza (olaparib). Prescribing information.AstraZeneca Pharmaceuticals.Revised January 2018.Accessed August 30, 2022. https://bit.ly/3wG2KJ8
10. Zejula (niraparib). Prescribing information.GlaxoSmithKline. Revised April 2020. Accessed August 30, 2022. https://bit.ly/3pVn3OW
11 .Li N, Zhu J, Yin R, et al. Efficacy and safety of niraparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer using an individualized starting dose (PRIME Study): a randomized, double-blind, placebo-controlled, phase 3 trial. Presented at: 2022 SGO Annual Meeting on Women’s Cancer; March 18-21, 2022. Phoenix, Arizona.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More