Roundtable Discussion: Hutson Addresses Challenges in IO-TKI Combination in Clear Cell RCC
During a case-based roundtable event, Thomas Hutson, DO, PharmD, discussed challenges with treating a 59-year-old woman with clear cell renal cell carcinoma.

Thomas Hutson, DO, PharmD


HUTSON: Are you all doing IMDC [International Metastatic RCC Database Consortium] risk calculations?1 Are you doing eyeball tests? Do you have electronic medical records [EMRs] that are making you do certain things? Or do you need to do anything additional?
XIONG: We don’t have this incorporated into our EMRs, so in day-to-day practice, a patient’s performance status trumps everything else. I’m aware of these risk calculators, and to me, they’re more for understanding clinical trials. I’m not sure how much that helps me in day-to-day practice.
GUO: We use iKnowMed, but not everybody uses this calculator [and] we’re not required to use that. I think most of us just use eyeball testing.
HUTSON: So it’s not built in? If you’re using iKnowMed—with whatever version you’re using—when you pull up your regimens, you’re not having to check these boxes? We are, in our iKnowMed.
GUO: We just started last year. We’re still trying to optimize the EMR system. It’s not totally done yet, and it’s not required at this time.
HUTSON: OK, fair enough. It sounds like some [people] don’t have to do that yet, and they’re not on a pathway yet. Does anyone have any thoughts as to whether they would be initiating systemic therapy?
MALIREDDY: The IMDC risk calculator helps me decide what category the patient falls into, then their median survival and everything [else factors in]. I know certain drugs are approved for certain risk categories, [which is] why I would use the IMDC risk calculator. It’s part of Texas Oncology, so we have to use it to get to the pathway. In this patient, I would start treatment.
HUTSON: I agree [and would start therapy, as well]. I think they give us some sizes to make us feel that this is a bulkier disease. They are an intermediate-risk patient, and the decision—multiple sites of metastases, somewhat bulky—is to prompt us to move forward with systemic therapy [in this case].
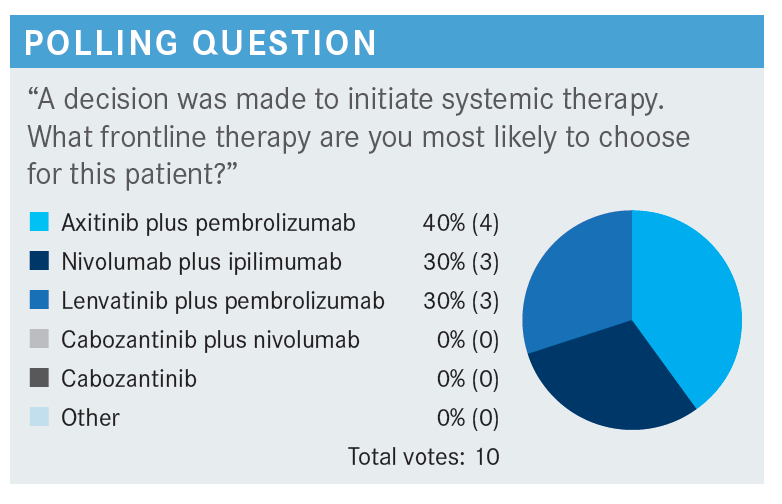
HUTSON: [In this poll], I chose lenvatinib [Lenvima] and pembrolizumab [Keytruda], but this is an area where all these agents listed are category 1 options in the National Comprehensive Cancer Network guidelines, so it becomes your opportunity to choose.2 With some, we have certain guidance. For instance, we have preferred regimens from US Oncology [Network] and McKesson [Corporation], and those are the pembrolizumab-containing regimens. We tend to go with the lenvatinib and pembrolizumab or axitinib [Inlyta] and pembrolizumab, but there’s nothing that prevents us from ordering any of the other options. Certainly ipilimumab [Yervoy] and nivolumab [Opdivo] becomes an option, as well.

CEN: I use [lenvatinib] and pembrolizumab a lot [with] my patients. So far, I have 5 patients on this regimen. It’s well tolerated. I would say…most of my patients need to dose reduce. I have a habit to tell my patient ahead of time [that] if you take 2 pills once a day and you get [adverse events (AEs)], then just reduce to 1 pill once a day, which is 10 mg daily. [I also tell them to] call us about [their] AEs. That way, most of my patients can continue the treatment without much discontinuation.
I do have other patients for whom I don’t use this regimen and use nivolumab and ipilimumab. I have 2 patients in whom the tumor invaded into the duodenum and who have a bleeding history. For those 2 patients, I use nivolumab and ipilimumab. We also have a clinical trial here on nivolumab and ipilimumab followed by cabozantinib [Cabometyx] vs placebo with the nivolumab.4
HUTSON: Is that the [ongoing] PDIGREE trial [NCT03793166] that you’re doing?
CEN: Yes, the PDIGREE trial.
GUO: I’ve used this regimen only once after approval for RCC.5 It’s not an easy regimen, even though I tend to start it from low dosage. I didn’t have that experience with hepatocellular carcinoma and some other cancer types. This patient did not tolerate it well, despite dose reduction.
We had to take a break. His disease volume was getting smaller and he responded well, so at this moment, he’s only on [pembrolizumab]. I’ve had more experience with the axitinib and [pembrolizumab]. I don’t think [lenvatinib] is that much more difficult. Most patients are going to need a dose reduction anyway. It’s kind of expected.
HUTSON: How about your experience with lenvatinib and everolimus? Have you used the 2 oral drugs together?
GUO: No, I have not. One of my partners did, and when I was covering, I saw that patient. That patient had a lot of AEs, too.
HUTSON: [That’s] fair. So, you predominantly use axitinib and pembrolizumab as your go-to, then. Are you using ipilimumab and nivolumab at all?
GUO: Well, I used to use it a lot more, because a small percentage of patients can come off medication. I still use it for patients whom I don’t believe would tolerate a tyrosine kinase inhibitor [TKI] plus immunotherapy [IO], or those patients who don’t have a rapidly progressive disease and I can afford waiting a little longer to see a response. So, I still use it if the right patient comes along.
HUTSON: That’s fair. [How about] you, Dr Malireddy?
MALIREDDY: Yes, I do use [lenvatinib] and pembrolizumab. I used it only on 1 patient, but all these years, I’ve been using pembrolizumab and axitinib a lot compared [with] this. [However], even with axitinib, I had to dose decrease a lot. Within the first month [of lenvatinib], we had [to decrease] the dose, which [was] expected. In the past, I used everolimus and lenvatinib, but in the later lines of treatment, we had a lot of toxicity in that setting.
HUTSON: What is everyone’s impressions of the CLEAR trial data?
MALIREDDY: I never compared them [myself], but to tell your patient 94% of the time you are going to show some response is impressive.6,7 It’s only 5% of the time that you are not going to. The patient may fall in that 5%, but that’s still impressive. Hopefully with the long-term data, we’ll see more [improvement].
SASHITAL: I haven’t used lenvatinib and pembrolizumab yet. I mostly use axitinib with pembrolizumab. I haven’t had any patients since. The main problem I face is hypertension. Most of the patients have severe renal insufficiency before; they already had a nephrectomy. So, you end up dose reducing with the hypertension, [and they’re] already on multiple antihypertensives. In fact, sometimes I have to give them a whole month off to get them better controlled, or the nephrologist will panic.
HUTSON: Are you starting them at full dose, then going down if they need it?
SASHITAL: No, I start at a lower dose and go up as tolerated.
HUTSON: Are you using any ipilimumab and nivolumab?
SASHITAL: I do. I have a couple patients. When they’ve had mixed histology and it wasn’t as progressive [of] a disease, I’ve used ipilimumab and nivolumab.
HUTSON: Do you choose what you’re using first because of what you want to use second? Or do you not worry about that and just give them [what you think is] the best?
SASHITAL: I like to keep cabozantinib as a second-line option, so I’m not using [the] cabozantinib and nivolumab option.
SOLANKI: The attractive [thing] about lenvatinib and pembrolizumab is the very high response rate. It’s particularly appropriate for patients who have large-volume symptomatic disease [who you] want a quick response in. [During our chemotherapy board discussions], we have considered using it for patients who present with a large tumor with or without metastases. The problem is that the primary doesn’t respond as well, but it still responds [approximately] 25% of the time.
My concern with lenvatinib is that the company has been slowly escalating the dose with each trial. They started with 14 mg, [then] they went to 18 mg, then to 20 mg, and 20 mg became a very hard dose to tolerate. The question I have for you is: At what point was the dose [reduction] required in terms of time from start? I’m wondering [whether] it might not be better to have an induction course, then move to a lower dose so you never have to watch for things. Do we have those data?
HUTSON: No, but at least with the data that have been done, and they’ve looked at this—in endometrial carcinoma, we’ve looked at it with lenvatinib and everolimus. The best practice is to truly start at full dose and let the AEs declare themselves. Even if it’s just 4 weeks and the patient is starting to say, “You know what? I’m having bad diarrhea. I can’t control it with loperamide [Imodium].” Then you go down to a lower dose, because it’s something about getting that [maximum concentration] and inhibiting [fibroblast growth factor], which is one of the things it’s inhibiting, and doing that to get the best result.
There were a couple studies [that looked at this]. For instance, [one study asked whether] we get a better outcome by starting at 14 mg. That study showed the outcome was inferior to starting at 18 mg and going down to 14 mg when needed.8 [Bottom line]—it’s tempting to want to start at a lower dose, but remember, it’s very hard to go up [From the Data8].
There are 20% to 30% of patients who need to be at the higher dose. There are those patients who don’t need to lower the dose, and you’d never find out who that one-third of patients are unless you go with [the] full dose first. I try to always encourage people to try to do that, but that’s not an absolute 100% of patients. There are always going to be [patients] you see [who] are especially frail, and you start off at a lower dose. Otherwise, I try to start [patients] at full dose, with an expectation that I’ll try to get them to the first scan in 3 months if I can. If we can’t make it because of the AEs, then we go to the lower dose, and that’s fine. That’s the best practice.
SOLANKI: That makes sense. I was just wondering [whether] there was a particular time during treatment where most people end up having to have dose reductions.
HUTSON: Yes, 3 to 6 months; it’s declaring itself then. You’ve got 2 waves of the dose reductions. You’ve got the initial, [where] it accumulates in the body and they’re dealing with those acute AEs they can’t control. Then there’s the group of patients who make it through that, but they get the second wave of chronic AEs that just gets to be too much.
SOLANKI: With all the [IOs], starting with interferon and interleukin-2 [Proleukin], the primary tumor never responded. That was true for sunitinib [Sutent], as well. Why is that?
HUTSON: That’s a good question. We don’t know. You do get some shrinkage of the primary tumor, but ideally, you [must] consider [those] who are the true complete responses [CRs].
For instance, [patients on] ipilimumab and nivolumab who are getting off therapy with no disease, or [those] who have had their kidneys removed. It’s hard to know why that is, but that is true. You do get some shrinkage of the primary tumor with it, but you’re right. It’s not going to put you into a CR if you have that.
SOLANKI: That’s right. It’s not 71% in the primary tumor.
XIONG: I haven’t used lenvatinib in any combination for [patients with] RCC, so I [must] say my go-to regimen is still axitinib and pembrolizumab.
HUTSON: How about lenvatinib and everolimus? Have you used lenvatinib and everolimus before in the refractory setting?
XIONG: This is the regimen I’m probably going to use next week. I already got the patient scanned. Right now, the patient is on axitinib and pembrolizumab and has progressive disease. The patient also had cabozantinib before, so that’s the regimen I’m probably going to use next for this patient.
HUTSON: The same management factors apply. As you get experience using the lenvatinib and everolimus, it’s trying to start at the full dose [and] lowering when you can. [You can use] those same principles.
XIONG: Lenvatinib is a peculiar agent. For different indications with different dose ranges, it’s confusing.
HUTSON: Yes, the dose levels are different in the different malignancies. A lot of it is because they try to maximize the most they can to get that inhibition, [as] they are inhibiting these other receptors.
XIONG: Yes, that we may come back to—not to the advantage of the company. We’ve always maximized the dose, but sometimes you [must ask]: What is the minimum effective dose? Maybe that’s the way to go.
HUTSON: It’s interesting, though. Remember, one-third of patients need the higher dose, so it’s not everyone that gets down. There are still the 30% of patients you’ll have at the high dose, and you’ll be able to hold things.
JANA: I predominantly use ipilimumab and nivolumab. I started using that right from the first approval. I like the fact that it has long-term data. [Although] I do think a TKI plus [IO] is an excellent option, I don’t see the difference between these various regimens enough to change my practice since I first started using that. More recently, in a couple patients, I started using everolimus plus the lenvatinib in the refractory setting.
HUTSON: Does anyone have any experience with using pembrolizumab and nivolumab? What are your impressions of it? Do they differ from what your impressions were with lenvatinib and pembrolizumab?
JANA: I have used nivolumab and pembrolizumab, and I don’t think it is any different from the toxicity perspective. I don’t have the numbers to compare the responses between these 2.
ALOBA: I have used cabozantinib and nivolumab, but I feel that cabozantinib is the hardest TKI to use among the 3. [Lenvatinib] is a little easier than cabozantinib. Cabozantinib as a single agent is difficult. Cabozantinib and nivolumab is also difficult. I would rather use [pembrolizumab] and [axitinib] or [pembrolizumab] and [lenvatinib] rather than cabozantinib and nivolumab.
HUTSON: Does anyone have any other experiences with it?
MALREDDY: I’ve had similar experience with cabozantinib and nivolumab. The [gastrointestinal] toxicity, dysplasia, and stomatitis [were] much higher with the combination. That’s the only time I’ve used it. Overall, I’ve been using the other combination regimens. Pembrolizumab, axitinib, then come down to cabozantinib alone in the second line.

HUTSON: Looking at those 2 more recent things in general, [does anyone] want to throw out what their reasons [are as to] why they are choosing what they’re choosing? What is your strategy? Do you have a strategy on handling AEs?
MALREDDY: The key factors in influencing frontline therapy are age, comorbidities, performance status, and the IMDC risk scoring. Basically, looking at the pathways and which drug is likely going to be approved through the insurance. These are all the things I [consider].
HUTSON: Does anyone just use 1 type of IO-TKI, and that’s their IO-TKI to use? Or do you go back and forth between multiple regimens?
MALREDDY: I saw a patient today who had a nephrectomy in 2014, then had metastatic disease to only the spine, with an L1 compression fracture. But he has psoriatic arthritis, [is] on multiple immunomodulatory agents, and apparently had uveitis related to that autoimmune disease. So I was not going to use the [IO] in this setting, I was going to use [cabozantinib].
HUTSON: The next [topic I want to address] is financial toxicity. [Are there] any issues there? How do you assist patients? Do you have any issues with the financial toxicity of the regimens? [Are there] any regimens [that are] easier than others?
SASHITAL: With any TKI, the problem is getting assistance. For many of the [health management organizations], by the time they get assistance, it takes [over] a month to get it. I try to get the free drugs for the first month, but then many of them are common supplemental insurances, so they don’t get the free drug. That sometimes makes the decision to go for ipilimumab and nivolumab.
HUTSON: What’s the average time that it takes to get an IO-TKI?
SASHITAL: Three weeks, at least.
HUTSON: For me, it’s [approximately 1] week. Is there anyone else who is having to wait a couple weeks to get an IO-TKI?
SASHITAL: When they go through the assistance system, patients are to give their financial information. They take time to reply, then, logistically, to get their tablet in hand, it takes time.
HUTSON: I’d certainly call the company, because, at least with Texas Oncology, our pharmacy group is very active.
REFERENCES
1.International mRCC Database Consortium. IMDC. Accessed August 20, 2022. https://bit.ly/3RjwDY8
2.NCCN.Clinical Practice Guidelines in Oncology. Kidney cancer,version 4.2022. Accessed March 30, 2022. https://bit.ly/2TAx1m3
3.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med.2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
4.Zhang T, Ballman KV, Choudhury AD, et al. PDIGREE: anadaptive phase IIItrial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). J Clin Oncol. 2021;39(suppl 6):TPS366. doi:10.1200/JCO.2021.39.6_suppl.TPS366
5.FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. August 11, 2021. Accessed September 1, 2022. https://bit.ly/3Rd1Xrk
6.ChoueiriTK, Eto M, Kopyltsov E, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Ann Oncol.2021;32(suppl5):S678-S724. doi:10.1016/j.annonc.2021.08.056
7.Grünwald V, Powles T, Kopyltsov E, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. J Clin Oncol. 2021;39(suppl15):4560. doi:10.1200/JCO.2021.39.15_suppl.4560
8.Pal SK, Puente J, Heng DYC, et al. Assessing the safety and efficacy of two starting doses of lenvatinib plus everolimus in patients with renal cell carcinoma: arandomized phase 2 trial. Eur Urol. 2022;82(3):283-292. doi:10.1016/j.eururo.2021.12.024

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen