Sabari Examines Frontline and Second-Line Treatment Options in NSCLC
Joshua K. Sabari, MD, goes through treatment options in the frontline and second-line for patients with non–small cell lung cancer in a Targeted Oncology case-based peer perspectives live discussion.

Joshua K. Sabari, MD, went through treatment options in the frontline and second-line for patients with non–small cell lung cancer in a Targeted Oncology case-based peer perspectives live discussion. Sabari, an assistant professor in the Department of Medicine at NYU Langone Health, explained the treatment options in the context of 2 patient cases of lung cancer.
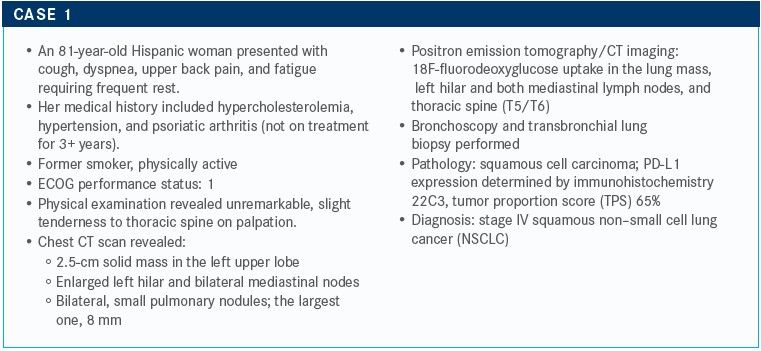
TARGETED ONCOLOGY: Do you order molecular testing for patients with squamous cell carcinoma?
Sabari: I order liquid biopsy and tissue biopsy tests. Liquid biopsy is controversial in this setting, but I order these tests to identify alterations. The key takeaway point here is that in squamous cell carcinoma, the rate for identifying an actionable mutation is a lot lower [rate than in other lung cancers]. There is lots of research going on in the space. [In general], the rate of EGFR [mutations] in squamous cell carcinoma is low, in the 1% range.
There are mutations that investigators may eventually develop treatments for, and which are undergoing development. There are also some other alterations that we can identify, but these don’t have an FDA-approved therapy available yet; however, I’m an optimist, so I still order a test.
I have a patient now who immigrated from China about 3 years ago. He never smoked but developed squamous cell cancer. He has an EGFR exon 19 [mutation]. Interestingly, he developed a C797S secondary mutation [while being treated on] Tagrisso [osimertinib] and is now doing quite well in a clinical trial. But the take-home point is that mutations are relatively rare.
TARGETED ONCOLOGY: What are your treatment options for this patient?
Sabari: We could go with a combination of chemotherapy and immunotherapy here, or even immunotherapy alone. To address the dyspnea with the back pain, we could also treat with radiation therapy up front, followed by immunotherapy.
TARGETED ONCOLOGY: What do the National Comprehensive Cancer Network (NCCN) guidelines recommend?
Sabari: This patient has a PD-L1 expression ˃≥50%. According to the guidelines, the preferred regimens are pembrolizumab [Keytruda] monotherapy, based on KEYNOTE-024 [NCT02220894], which compared pembrolizumab with chemotherapy.1,2
The other option recommended is carboplatin, paclitaxel, or albumin-bound paclitaxel [Abraxane], plus pembrolizumab, based on KEYNOTE-407 [NCT02775435].3 Another option for certain cases is the combination of ipilimumab [Yervoy] and nivolumab [Opdivo], based on CheckMate 227 [NCT02477826].4
KEYNOTE-024 evaluated pembrolizumab versus platinum-doublet chemotherapy in the frontline setting in a total of 305 patients who had NSCLC, either squamous cell carcinoma or adenocarcinoma. Patients then received pembrolizumab maintenance therapy. This was the first study that led to the approval of any immunotherapy in the frontline setting for NSCLC.
Median progression-free survival [PFS] in the immunotherapy arm was 10.3 months versus 6 months for the control arm [HR, 0.50; 95% CI, 0.37-0.68; P <.001]. The results led to the FDA approval [for pembrolizumab] for patients with metastatic NSCLC whose tumors express PD-L1 as determined by an FDA-approved test in October 2016.5 At that time, the overall survival [OS] benefit had not been reached; but now we have 5-year data coming up, which continue to show the improved OS for pembrolizumab over chemotherapy. We also check PD-L1 expression status in patients because of this clinical trial.
This study demonstrated the role of sequencing, which is a somewhat controversial idea. It demonstrated that giving pembrolizumab first, before chemotherapy, resulted in benefit.
There was a cure rate of about 25%. Some of the patients never went on to chemotherapy, but about 75% of patients did receive chemotherapy over the course of treatment. As a result, the crossover data continue to accrue. OS still favored the pembrolizumab arm versus the chemotherapy arm in patients who had PD-L1 expression ˃≥50%.
We have more prospective data across multiple studies that suggest immunotherapy can be stopped after 2 years. There have been some relapses, but most patients continue to respond.
TARGETED ONCOLOGY: Is there a reason to focus on those patients who benefitted from pembrolizumab?
Sabari: We never talk about cure. We talk about no evidence of disease, but that’s the billion-dollar question, and I just don’t know. I’m sure there’s a biologic or immunologic mechanism, but we don’t know what that is.
TARGETED ONCOLOGY: How do the results of the KEYNOTE-407 trial help you when treating a patient such as the one described?
Sabari: This study specifically focused on patients with squamous NSCLC.6 This was a large study that randomized patients 1:1 to pembrolizumab plus chemotherapy versus placebo plus chemotherapy. The investigators allowed for optional crossover, which will have an eff ect on OS results.
Administering carboplatin every 3 weeks was a concern because of toxicity, but patients seem to tolerate Abraxane on a weekly basis. My patients with squamous NSCLC tend to be in rougher shape than my patients with adenocarcinoma, so I tend to avoid this. I use this dosage more in the neoadjuvant or adjuvant setting in patients with squamous NSCLC who are potentially curable.
The investigators conducted a second interim analysis, looking at PFS stratified by PD-L1 expression subgroups. The median PFS was 6.4 months versus 4.8 months favoring the pembrolizumab arm [HR, 0.56; 95% CI, 0.45-0.70; P <.0001].
Patients were stratified by TPS <1%, TPS 1% to 49%, and TPS ≥50%. Patients with a TPS <1% demonstrated a median PFS of 6.3 months versus 5.3 months favoring the pembrolizumab arm [HR, 0.68; 95% CI, 0.47-0.98]. Patients with TPS ≥50% did quite well, however.
My standard of care has been this regimen described in [the] KEYNOTE-407 [trial] in my patients with squamous NSCLC, regardless of PD-L1 expression and depending on the patient’s performance status. If patients have a poor performance status [score], I’m going to administer pembrolizumab alone. If they have good performance status and a high PD-L1 expression, I will offer this regimen because I think the data are better [in this population].
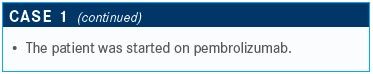
TARGETED ONCOLOGY: How does the patient’s age and performance status factor into your treatment choice?
Sabari: Age is just a number. I think what matters is performance status [score]. In the ideal world, you conduct a geriatric assessment, or refer the patient to a geriatrician, but sometimes I just walk the patient to the secretary’s desk to make the appointment. If the patient is able to get up and walk with me and the patient tells me that they are active, that’s my assessment. In an ideal world, we would send them for the assessment.
TARGETED ONCOLOGY: How does the patient's psoriatic arthritis affect your treatment decision?
Sabari: This is an underlying autoimmune disease. This condition might give a clinician a reason to pause. But I think the consensus is that unless the patient is on high-dose disease-modifying antirheumatic agents, they tend to tolerate immunotherapy.
A retrospective study from the Dana-Farber Cancer Institute evaluated patients with different autoimmune diseases with NSCLC who receive PD-L1 monotherapy.7 Eighteen percent of patients had an autoimmune-related disease, and 20% received immunomodulatory agents for their autoimmune disease. The key takeaway is that only a minority of patients experienced an exacerbation of their autoimmune disease.
There’s an ongoing study by Bristol Myers Squibb analyzing therapy that includes nivolumab. The company is enrolling patients who have no autoimmune disease and is looking at the rate of toxicity. Incidence of autoimmune or immune-related adverse events [AEs] was similar to that reported in the clinical trials, which did not include patients with an autoimmune disorder.
Additionally, we now have data in melanoma that has come out of Memorial Sloan Kettering Cancer Center that evaluates patients who experience autoimmune AEs from immunotherapy and then were re-treated. The response rate is right around that 20% to 25% range. The rate of immune-related AEs is a bit higher in patients who have previously experienced AEs.
The investigators found that it was safe to re-treat patients, with the exception of [those who had] pneumonitis. That was the 1 [group] that was potentially not safe. But overall, I think it is safe to treat our patients who have underlying rheumatologic or autoimmune disease, [if they are not currently treated with] disease-modifying agents.
It’s important to be able to seek out a rheumatologist, endocrinologist, or a dermatologist. Having a diverse group of care managers taking care of your patients with you [is beneficial] because patients can run into issues with immunotherapy. We have become more cognizant of that, but I think it’s important to have those consultants available should we need them.
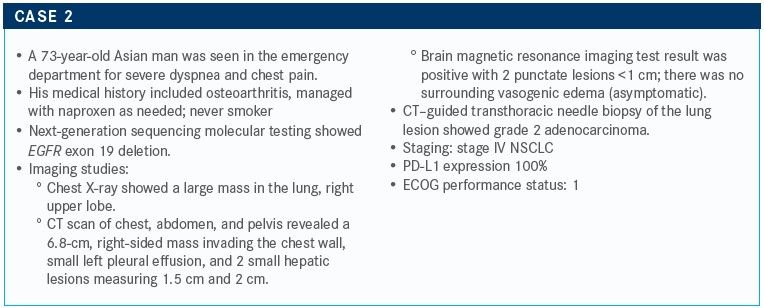
TARGETED ONCOLOGY: What molecular testing should be ordered? Would you initiate frontline therapy or wait for molecular testing results?
Sabari: I would order a large panel test. I think in someone who is a never smoker, we have a high pretest probability of having a driver alteration. I would recommend waiting for the results of that test before moving forward. However, if the patient is symptomatic, I don’t think it’s wrong to start chemotherapy alone. It’s hard for me to sit on my hands when I see a sick patient. I will give chemotherapy and hold off on immunotherapy. You can always continue the chemotherapy, depending on the situation.
TARGETED ONCOLOGY: What would you consider as frontline therapy for this patient?
Sabari: EGFR occurs in about 20% to 25% of patients. About 30% to 35% of my patients have an EGFR mutation, given my patient population in downtown Manhattan.
In the frontline setting, there is the first-generation EGFR inhibitor Tarceva [erlotinib]. Second- generation inhibitors include gefitinib [Iressa] and afatinib [Gilotrif]. The third-generation inhibitor is osimertinib. All those agents are approved in the frontline setting, and osimertinib is the newest therapy available in the frontline setting.
TARGETED ONCOLOGY: Is it preferable to use osimertinib up front, or should its use be delayed because of the potential for resistance?
Sabari: I learned early on in my training to use your best drug up front. I think that osimertinib is a better drug than the first- and second-generation EGFR inhibitors. Now that we have OS data for Tagrisso, I think it makes sense to use Tagrisso in the frontline setting and not to hold it in your back pocket because the patient could develop an EGFR T790M mutation. Forty percent of patients will not develop T790M.
The space is getting complicated, but I’m still using Tagrisso as a single agent as the preferred treatment.
TARGETED ONCOLOGY: For a patient with a sensitizing EGFR mutation in exon 19 or exon 21, what treatment options do the NCCN guidelines suggest?
Sabari: The [NCCN] guidelines suggest Tagrisso as a preferred regimen.8 Other recommended agents include erlotinib and ramucirumab. I did not mention dacomitinib [Vizimpro]. That’s also a second-generation EGFR inhibitor available in the frontline setting as well.
TARGETED ONCOLOGY: Please provide details about the FLAURA trial (NCT02296125).
Sabari: This study evaluated the fi rst-line use of the third-generation EGFR inhibitor, Tagrisso versus standard of care, which was erlotinib or gefitinib. Interestingly, the investigators excluded afatinib, which is a second-generation EGFR inhibitor that we know is better than these 2 other drugs.
The primary end point was PFS, which was 18.9 months for the Tagrisso arm compared with 10.2 months for the standard-of-care arm [HR, 0.46; 95% CI, 0.37-0.57; P <.0001].9 The OS results were published in the New England Journal of Medicine.10 The investigators reported a 39-month OS for the Tagrisso arm versus 31.8 months for the standard-of-care arm.
One thing to point out was that patients who initially received erlotinib or gefitinib were able to cross over to receive osimertinib. Overall, I think this was a win for patients with an EGFR mutation. I think we’re getting better at treating these patients with EGFR mutations.
Of interest is that patients could cross over, but only if they had T790M [sensitizing mutations]. If patients were in the population who did not have T790M, which was about 40%, there was no benefit giving Tagrisso.
TARGETED ONCOLOGY: What is the role that sequencing plays in efficacy with these multigenerational tyrosine kinase inhibitors for this patient population?
Sabari: We still need to better understand sequencing, but we can’t do that comparing across clinical trials. We will need a better biomarker that identifi es who develops T790M and who doesn’t. But we do know that patients who take Tagrisso develop secondary resistance mechanisms like MET or C797S resistance mutations. There are multiple ongoing trials.
There’s a clinical trial that evaluates the bispecific antibody JNJ-372 in which we are seeing responses in the 40% to 45% range in patients who have MET amplification post-EGFR inhibitor [NCT02609776]. I believe there will be potential strategies [in the future] that combine different antibodies with EGFR inhibitors or strategies that combine chemotherapy with EGFR inhibitors.
TARGETED ONCOLOGY: Why do these mutations tend to develop in Asian populations?
Sabari: We don’t exactly know [why], but the EGFR mutation does seem more common in Asian patients, particularly, Asian women. It is not clear why. Examining the subset populations suggests that there is a difference between exon 19 deletion and L858R. The latter seems to have a worse prognosis, which we see in patients of Asian descent versus non-Asian descent. But we don’t have a rationale for this observation.
I’ve examined a small subset of 400 patients at New York University but could not tease out any major difference, including coalteration, driver mutations, or any sort of criteria where patients did better or worse. The only thing that seemed different was smoking status. If a patient was a smoker or if they had a TP53 coalteration with EGFR, they tend to do worse with osimertinib, but not with first- and second-generation EGFR inhibitors.
Interestingly, pneumonitis and inflammation in the lungs tends to be higher in patients of East Asian descent, but not in Caucasians. But we don’t know why there is a difference. It could be pharmacodynamics because there might be a difference in how subpopulations break down drugs.
TARGETED ONCOLOGY: What other treatments are available for EGFR-mutated NSCLC?
Sabari: The RELAY study evaluated erlotinib with or without ramucirumab [NCT02411448].11 Investigators looked at treatment until progression or unacceptable toxicity. The primary end point was PFS. Patients were stratifi ed by exon 19 versus exon 21 L848R, by sex, region, East Asian descent, and by EGFR testing method.
Investigators reported that the PFS primary end point was 19.4 months for patients who received the combination compared with 12.4 months in the control arm [HR, 0.591; 95% CI, 0.461-0.760; P <.0001]. The PFS for osimertinib in the RELAY trial was reported as 18.9 months. The PFS rate was 71.9% in the treatment arm versus 50.7% in the control arm.
When toxicity was evaluated, the only treatment-related AEs [TRAEs] that stood out were related to VEGF inhibition. For patients in the combination arm, the grade 1/2 rate for hypertension was 22% compared with 7% in the control arm. For grade 3 hypertension, the combination arm was 24% versus 5% in the control arm.
Most clinicians who treat with VEGF inhibitors are used to managing hypertension, but the investigators noted that grade 1 bleeding and hemorrhaging was higher in the ramucirumab group [53%] versus 24% in the control arm. Overall, grade 3/4 TRAEs were not too severe. This is another regimen to consider when discussing treatment options with your patients.
I think we need to consider combination strategies and that’s why I think these studies are important.
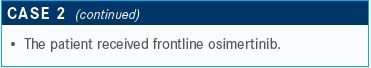
TARGETED ONCOLOGY: What are the potential treatments in the next-line setting?
Sabari: We have data evaluating carboplatin and pemetrexed [Alimta], but we don’t have data for pembrolizumab in the second-line setting. I would, however, mention that I would avoid pembrolizumab in the second-line setting because it can prevent your patient from receiving other targeted therapies [in the future].
References
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 3.2020. Published February 11, 2020. Accessed April 6, 2020. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
2. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi:10.1056/NEJMoa1606774
3. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi:10.1056/NEJMoa1810865
4. Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137-147. doi:10.1016/j.ejca.2019.05.008
5. Pembrolizumab (KEYTRUDA) checkpoint inhibitor. News release. FDA Office of Media Affairs; October 24, 2016. Accessed April 6, 2020.www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-checkpoint-inhibitor
6. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi:10.1056/NEJMoa1810865
7. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36(19):1905-1912. doi:10.1200/JCO.2017.77.0305
8. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer (version 3.2020). Published February 11, 2020. Accessed March 30, 2020. nccn.org/professionals/physician_gls/pdf/nscl.pdf
9. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
10. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41-50. doi:10.1056/NEJMoa1913662
11. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655-1669. doi:10.1016/S1470-2045(19)30634-5
