Ilson Explores Treatment Options for Patients With Metastatic HCC
In a Targeted Oncology case-based peer perspectives live discussion, David H. Ilson, MD, PhD, discusses the treatment options for patients with hepatocellular carcinoma
David H. Ilson, MD, PhD

In a Targeted Oncology case-based peer perspectives live discussion, David H. Ilson, MD, PhD, medical oncologist, Memorial Sloan Kettering Cancer Center, New York, NY, discusses the treatment options for patients with hepatocellular carcinoma (HCC).
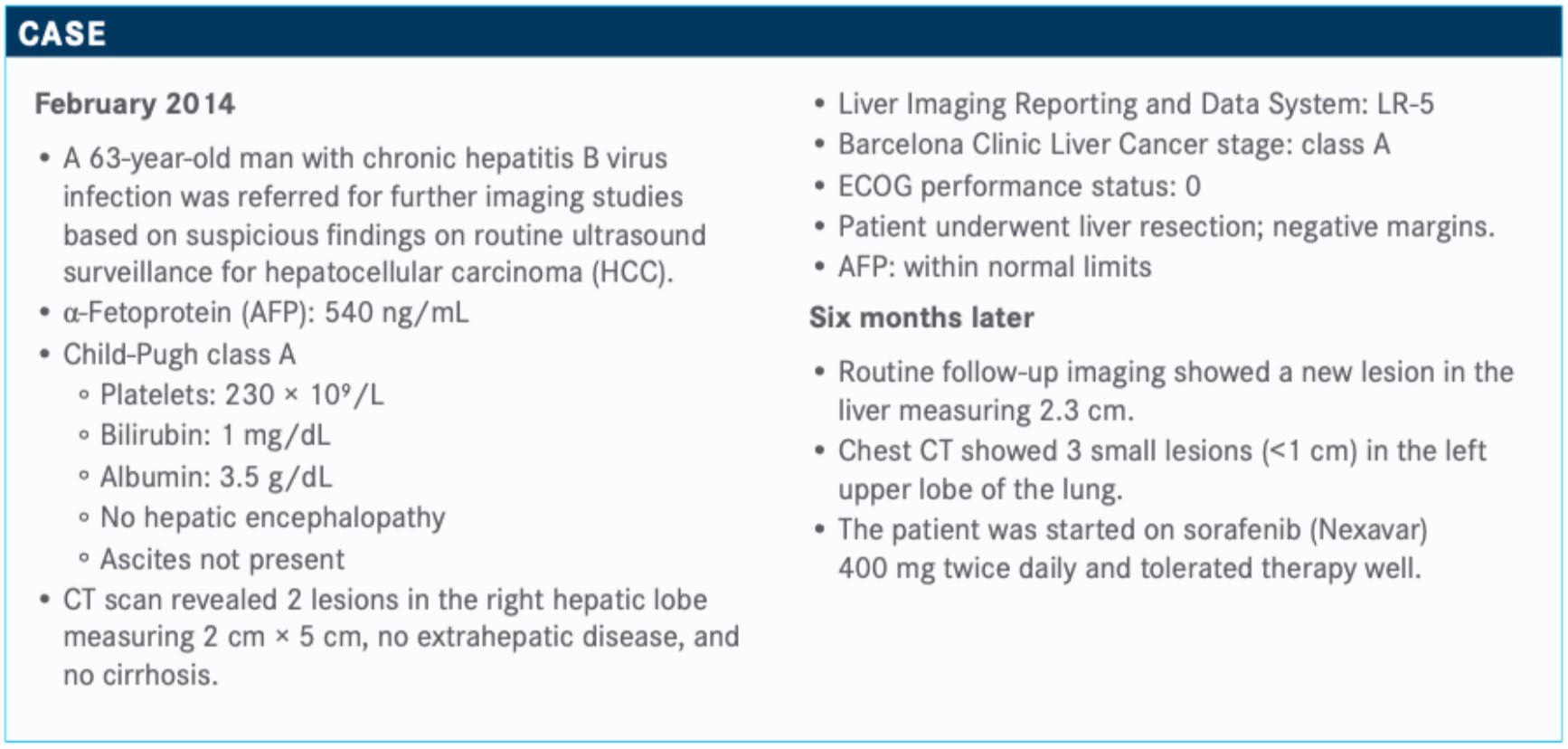
Targeted Oncology: Would you consider a liver transplant for this patient? What are the frontline options in this setting?
ILSON: Liver transplant is reserved for patients with HCC who have liver dysfunction. In a patient with a healthy liver, hepatic resection is the up-front treatment for this disease, and he under-went hepatic resection.
Then, 6 months later, he had metastatic disease and was started on standard sorafenib 400 mg twice daily and toler-ated it well. Alternatives to sorafenib in the first line [are] lenvatinib [Lenvima], which is also a multitargeted tyrosine kinase inhibitor.
Please describe the data for these 2 therapies.
This was the subject of the REFLECT trial [NCT01761226], a large, randomized trial in nearly 1000 patients with untreated HCC. Stratifi cation included the region they were treated, and they were randomized 1:1 to lenvatinib versus sorafenib with a primary end point of overall survival [OS]. The investigators were trying to demonstrate that lenvatinib was noninferior to sorafenib. Secondary end points were progression-free survival [PFS], time to progression, and response rate.1
Lenvatinib was noninferior to sorafenib in OS, but there were trends suggesting better PFS for lenvatinib, better time to progression, and a higher response rate for lenvatinib over sorafenib. But in terms of the primary end point, lenvatinib was noninferior; it was not superior with suggestion of a better PFS and response rate.Based on these data, lenvatinib has been approved.2 Adverse effects [AEs] with lenvatinib were more hypertension, diarrhea, and anorexia; there was incidence of more hand-foot syndrome and cutaneous toxicity with sorafenib. There was a suggestion of a longer treatment duration for lenvatinib over sorafenib and a slightly higher rate of discontinuation for AEs.1
What other drugs are being evaluated for this patient population?
The IMbrave150 trial [NCT03434379] recently reported results. This was first-line treatment with atezolizumab [Tecentriq] plus bevacizumab [Avastin], so a PD-L1 inhibitor plus bevacizumab versus sorafenib in first-line treatment.3
IMbrave150 may potentially be a practice-changing trial globally. Until the presentation of this trial, sorafenib was the standard first-line treatment for HCC. This was a randomized first-line trial in 500 patients with HCC without previous systemic therapy. The stratification was by region, performance status, and AFP. The standard arm was sorafenib 400 mg twice daily, and the investigational arm was the anti–PD-L1 drug atezolizumab every 3 weeks with bevacizumab every 3 weeks. There were 2 co-primary end points: OS and PFS.
How did atezolizumab and bevacizumab compare with sorafenib in the IMbrave150 trial?
There was a striking survival benefit for atezolizumab plus beva-cizumab versus sorafenib. The 6-month OS rate was 85% for atezolizumab and bevacizumab versus 72% for sorafenib. The median survival had not yet been reached on the experimental arm compared with 13.2 months for sorafenib. The hazard ratio was 0.58 [95% CI, 0.42-0.79; P = .0006], so that translates into over a 40% reduction in the risk of death. This was highly statistically significant. These data were presented at the European Society for Medical Oncology Asia 2019 meeting.
PFS was also improved with a median difference of about 2.5 months [6.8 months with atezolizumab and bevacizumab, 4.3 months with sorafenib]. There was a hazard ratio of 0.60 [95% CI, 0.47-0.76; P <.0001]...and a 40% reduction in progression, which is statistically significant. There was a significant portion of patients who were still progression free into the second year of treatment.
I suspect that regulatory approval for atezolizumab and beva-cizumab will be forthcoming. This is the first randomized trial [whose results] show a survival benefit above sorafenib. The response rate and disease control rates were also higher with atezolizumab and bevacizumab.

What treatment would you recommend for the patient considering his status at follow-up?
He has a high AFP [level] and progressed on sorafenib. There are a lot of options here: regorafenib [Stivarga], cabozantinib [Cabometyx], ramucirumab [Cyramza], nivolumab [Opdivo], and pembrolizumab [Keytruda]. All of these are options for this patient. In terms of the options that show a survival benefit in a randomized clinical trial, [there is] regorafenib.4
The RESORCE trial [NCT01774344] was a randomized trial with patients who progressed on sorafenib but tolerated it and had Child-Pugh A disease. Investigators looked at second-line regorafenib versus [placebo], and OS was the primary end point. This showed a significant improvement in median survival [10.6 months with regorafenib vs 7.8 months with placebo]. Median PFS was also modestly improved, and AEs were manageable.4
This is now an FDA-approved drug in the second line.5
What other drugs are approved in this setting?
The next approved drug in the second line is cabozantinib.6
CELESTIAL [NCT01908426] was a second-line trial in patients with Child-Pugh A disease. Patients with progression on first-line sorafenib were randomized 2:1 to cabozantinib, which is a meta-inhibitor, versus placebo. The primary end point was OS. There was no crossover.7
This was another positive trial of a second-line agent. Median OS was improved by about 2 months with cabozantinib, and there was improvement in disease control favoring the cabo-zantinib arm. There were few responses, though, only about a 4% response rate, but there was disease stabilization and improvement in PFS.
Ramucirumab in the second line [is also approved].8 The original REACH trial [NCT01140347] treated all patients with sorafenib progression, and this was a negative trial; however, in the subset of patients who had a high AFP [level], there was a survival benefit.9 This led to the REACH-2 trial [NCT02435433], which enrolled only patients on second-line therapy who had an AFP [level] higher than 400 ng/mL.10
How did ramucirumab do in terms of efficacy in the REACH-2 trial?
REACH-2 [results] showed a PFS benefit for ramucirumab over placebo [HR, 0.45; 95% CI, 0.34-0.60; P <.0001] and a modest survival improvement. The pooled data from these trials look at [the overall population and] classification of liver function. There were [survival] benefits as well as PFS improvement. This is another option for a patient with a high AFP [level]. A big plus with ramucirumab is that it’s nontoxic, so this is a drug I would consider in the second line for a patient with a high AFP [level] who is not symptomatic and for whom we may want to give something that is less toxic. Cabozantinib, arguably, is going to have more toxicity.
Would you consider checkpoint inhibitors such as nivolumab for this patient?
The phase II data for nivolumab in CheckMate 040 [NCT01658878] that led to approval had response rates of about 20% across the board, with response durations that were respect-able, about 8 to 9 months.11,12
There were provocative data for the combination of nivolumab and ipilimumab [Yervoy] in cohort 4 of CheckMate 040. This was more for second-line or refractory disease. This looked at nivolumab and ipilimumab in 3 different combinations followed by nivolumab maintenance.13
The regimen that had the best response rate was arm A, with nivolumab at 1 mg/kg and ipilimumab at 3 mg/kg every 3 weeks. There was the suggestion of best OS [for this regimen]. The caveat here is this was a phase II trial with small numbers, so there is a suggestion of some additive benefit for ipilimumab and nivolumab. They did not see a lot of grade 3/4 toxicity, even for the dual-targeted regimens.
Based on these data, the arm A regimen was FDA approved in second- and third-line treatments.14 I always pause when I see drugs approved based on second-line data, [although] this does represent a treatment option for later lines, but nivolumab plus ipilimumab is going to put the patient at risk for more toxicity.
The FDA granted approval for the combination in patients with refractory disease. That being said, however, the phase III trials of checkpoint inhibitors as single agents have been negative. In CheckMate 459 [NCT02576509], untreated patients received nivolumab versus sorafenib. There was no clear survival difference. PFS and OS were not improved. Nivolumab did have a better response rate, but this was a negative trial for first-line nivolumab versus sorafenib.15
What other studies looked at treatment for patients with HCC?
The second-line trial for pembrolizumab versus placebo in KEYNOTE-240 [NCT02702401]. These were patients progressing on sorafenib. There was a trend toward better survival for pembro-lizumab, but it was not statistically significant: 13.9 versus 10.6 months for pembrolizumab versus placebo, respectively [HR, 0.781; 95% CI, 0.611-0.998; P = .0238). It did not meet the criteria to declare pembrolizumab better than supportive care, even though there was a trend.16
The response rate was higher, 18% versus 4%, with pembrolizumab. The disease control rate was similar overall.
References
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in fi rst-line treat-ment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi:10.1016/S0140-6736(18)30207-1
- FDA approves lenvatinib for unresectable hepatocellular carcinoma. FDA. August 16, 2018. Accessed February 19, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma
- Cheng AL, Qin S, Ikeda M, et al. IMbrave150: effi cacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as fi rst treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(suppl 9):ix186-ix187. doi:10.1093/annonc/mdz446.002
- Bruix J, Qin S, Merle P, et al; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66. doi:10.1016/S0140-6736(16)32453-9. Published correc-tion appears in Lancet. 2017;389(10064):36. doi:10.1016/S0140-6736(16)32615-0
- Regorafenib. FDA. April 27, 2017. Accessed April 29, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/regorafenib
- FDA approves cabozantinib for hepatocellular carcinoma. FDA. January 14, 2019. Updated March 12, 2019. Accessed April 29, 2020. https://www.fda.gov/drugs/fda-approves-cabozantinib-hepatocellular-carcinoma
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54-63. doi:10.1056/NEJMoa1717002
- FDA approves ramucirumab for hepatocellular carcinoma. FDA. May 10, 2019. Accessed April 29, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ramucirumab-hepatocellular-carcinoma
- Zhu AX, Park JO, Ryoo BY, et al; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carci-noma following fi rst-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859-870. doi:10.1016/S1470-2045(15)00050-9
- Zhu AX, Kang YK, Yen CJ, et al; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282-296. doi:10.1016/S1470-2045(18)30937-9
- FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. FDA. September 22, 2017. Updated September 25, 2017. Accessed February 18, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepato-cellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi:10.1016/S0140-6736(17)31046-2
- Yau T, Kang Y-K, Kim T-Y, et al. Nivolumab (NIVO) + ipilimumab (IPI) combina-tion therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol. 2019;37(suppl 15):4012. doi: 10.1200/JCO.2019.37.15_suppl.4012
- FDA grants accelerated approval to nivolumab and ipilimumab combination for hepa-tocellular carcinoma. March 10, 2020. Updated March 11, 2020. Accessed April 29, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma
- Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as fi rst-line (1L) treatment in patients (PTS) with advanced hepatocellular carcinoma (AHCC). Ann Oncol. 2019;30(suppl 5):v874-v875. doi: 10.1093/annonc/mdz394.029
- Finn RS, Ryoo BY, Merle P, et al; KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193-202. doi:10.1200/JCO.19.01307CBPP_MAY2020.2 copy.indd 51CBPP_MAY2020.2 copy.indd 515/11/20 4:53 PM5/11/20 4:53 PM

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More