Roundtable Discussion: Sequencing and Tolerability Sway Treatment Choices for Patients With Clear Cell RCC
During a Targeted Oncology case-based roundtable event, Timothy M. Kuzel, MD, discussed the treatment options for patients with clear cell renal cell carcinoma.
Timothy M. Kuzel, MD (Moderator)
Samuel G Taylor III MD Professor of Oncology
Professor, Department of Internal Medicine
Division of Hematology, Oncology, and Cell Therapy
Rush Medical College
Chief, Division of Hematology, Oncology, and Cell Therapy
Interim Deputy Director
Rush University Cancer Center Chicago, IL

KUZEL: With a diagnosis of recurrent metastatic clear cell RCC, how do you assess risk? Is there a tool that you use?
SUMOZA: [We look] into the risk models and based on that, we decide what to use.
KUZEL: Do you find one easier to use than the other or have preferences?
SUMOZA: Probably the Memorial [Sloan Kettering Cancer Center risk model]; it…has been [around] for a longer period of time in the guidelines.1
KUZEL: It also has 1 fewer point to remember. The IMDC [International Metastatic RCC Disease Consortium] risk calculator is another alternative.2
The Memorial Sloan Kettering criteria was the first of the riskscoring models and was used in a large number of clinical trials to stratify patients….Basically the tools are designed to identify favorablerisk, intermediate-risk, and poor-risk patients. Some of our therapies are approved for 1 setting or another, so this becomes a valuable tool.
The IMDC is a web-based tool. It takes some of the memory and the thinking out of it. You can pop up the web-based tool, click in the patient’s clinical features, and it will then automatically calculate the number of features and give you the risk stratification.
In this case, based on this patient’s features—rapid recurrence risk, good performance status, mild anemia, and all other normal laboratory parameters—she has 2 risk factors, and she would be intermediate risk. We will move forward and think about what that implies for treatment here.
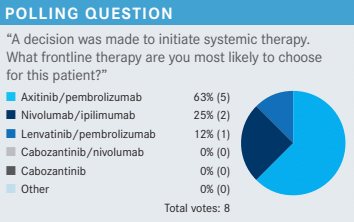
KUZEL: For those of you who chose axitinib [Inlyta]/pembrolizumab [Keytruda], is there any particular reason why that was your choice?
MULHERIN: I think it is fairly well tolerated. [Also,] the patient is 59 years old and she is in relatively good shape. I am anticipating she is probably going to survive into the second-line setting as many of these patients do, so I also think about planning out a sequence for what would I like to use next. I like to use cabozantinib [Cabometyx] as secondline therapy if they did not get it in the frontline setting. I probably tend to use nivolumab [Opdivo]/cabozantinib in patients who are frail; I think it is a bit easier. By comparison, I found lenvatinib [Lenvima] to be probably the most difficult TKI [tyrosine kinase inhibitor] of the 3 out there. So although I have used that, I have used it in the most symptomatic patients. You can look at the data and try to do crosstrial comparisons and all that, but that is just my experience.
KUZEL: For those who chose ipilimumab [Yervoy]/nivolumab, what is your rationale?
STANKIEWICZ: I chose nivolumab/ipilimumab because it can be time-limited therapy if she responds well. After ipilimumab is done, you can continue nivolumab, and if she is doing well at 2 years, maybe she can stop.
KUZEL: If you chose a combination with a TKI, do you continue it past 2 years if the patient is doing well?
STANKIEWICZ: I do not. It depends how they tolerate it. Sometimes you can drop the TKI even sooner and continue with the immunotherapy only.


KUZEL: [Has anyone] used lenvatinib/pembrolizumab? Often I find when I speak to community-based doctors, their exposure to lenvatinib tends to be more [with] thyroid cancer, or lenvatinib/pembrolizumab in other cancers. What is your expectation of toxicity?
CONTE: Yes, that sounds about right. It is not an easy medication. I heard from speakers in the gynecologic oncology world that they do not start at the full dose anymore. They now go in with 14 mg as the starting dose. Everybody has their own preference.
KUZEL: They have given up on the 20 mg altogether. That is interesting.
CONTE: One well-known speaker said that. Another speaker contradicted it.
KUZEL: Yes, that is a debate that you often will see. Is it better to start with a full dose and then have toxicity and dose reduce? Or is it better to start at a lower dose and then stay there? Unfortunately, there aren’t trials that answer those questions, so it becomes treater-dependent, and up to your philosophy. There is no doubt with lenvatinib, hypertension in particular can be a significant adverse event [AE]. Have you used lenvatinib at 20 mg in thyroid cancer? Do you find 20 mg is tolerable there?
MULHERIN: When I have used it in thyroid cancer, almost all of the patients have been younger. I have used 20 mg, but I had to dose reduce a fair number of those—not everyone. It is not the hypertension; we can usually deal with hypertension. I find it causes more fatigue, stomatitis, nausea, and anorexia. I think it is those vaguer things that tend to be more problematic.
KUZEL: When do you use lenvatinib/everolimus in this disease? Do you start at the full dose, which is 18 mg, or do you start with some sort of dose reduction if you use that combination? I know we heard [some clinicians] use cabozantinib second line. Do [clinicians] use lenvatinib/everolimus third line?
MULHERIN: After cabozantinib, but it’s hard.
KUZEL: What do you do after cabozantinib, [if] they are in the second line?
MULHERIN: If they start with nivolumab/cabozantinib, you might consider doing something [with lenvatinib] as second line. They are never going to be as fit as in the frontline setting. You want to use your most-effective regimen upfront. How likely is it that they are going to survive at secondline setting? The poor-risk patients, maybe not so much; an unhealthy minority will not. In the favorable and intermediate risk, it tends to be higher. So if I do nivolumab/ cabozantinib upfront, it tends to be in the frailer patients. But then I [use a lenvatinib combination] as second line and it is harder, and I probably dose reduce most of those patients to start with.
KUZEL: Is your choice of pembrolizumab/axitinib somewhat familiarity driven? It was the first combination [available].
MULHERIN: Sure, first-mover advantage.
KUZEL: You all learned to use it, so you keep using it to some extent. Overall, second-line therapy does drive your first-line choice, as well as just familiarity with these drugs.

KUZEL: The overall survival data in this trial were updated [HR, 0.70; 95% CI, 0.55-0.90].3 Does it change the way you think about this regimen? At 2 years, 70% of patients were still alive. Is this a regimen that can make you think twice about ipilimumab/nivolumab or pembrolizumab/axitinib? SHADE: My impression [is that] compared with pembrolizumab/axitinib, it is similar in terms of efficacy, but a little bit more toxic.
STANKIEWICZ: Especially for certain patients, the TKIs are quite difficult to take, and to continue to take. This is my impression. They have a quite a few AEs and patient [adherence] may be at question. Patients do not like [feeling] tired or having diarrhea and mucositis. All this is manageable, so it depends how patients are truly dealing with it—if they have support or not—but they are not easy to take. I know there are advantages of getting a better response rate, or hopefully this is going to translate into overall survival; but [CheckMate 9ER regimen] compared with sunitinib [Sutent] does not seem like it is a huge advantage in overall survival. It’s a few months.
SUMOZA: Tolerability and toxicity, we have to consider all this. Sometimes it is hard for some patients to tolerate this treatment.
KUZEL: Are you an ipilimumab/nivolumab fan, or pembrolizumab/axitinib?
SUMOZA: I am an ipilimumab/nivolumab fan.
KUZEL: Fair enough, avoiding the TKI if you can for a while. The dosing in the CheckMate 9ER trial: It’s important to remember that cabozantinib is only 40 mg in the combination arms.4 I think some [clinicians] remember 60 mg of cabozantinib as being a bit harsh, which is the single-agent dose, but it is only 40 mg in this combination. For those of you who have used this combination, do you think that makes it much easier? Or is it still a bit of a challenge?
CONTE: I think any dose reduction helps. In rural practices, where we have a lot of [older] patients in their 70s and 80s, they have a lot of AEs. They get upset; they will refuse to come back to the clinic. They will refuse anything if [it is too difficult] in the beginning. You have to talk them into treatment and then if they have all these AEs, they feel like you have betrayed them. I remember 10 years ago when we had none of these medications, we had many patients on single-agent pazopanib [Votrient], any age group, and many had very few AEs. Some had a good response. So I think in [older] patients, especially with good prognostic features, single agent is a possibility too.
SHADE: I agree with that….[For] the [older] patients, a lot of times I might start with a single-agent TKI and reserve checkpoint inhibitor for next line.
KUZEL: What is your preferred single-agent TKI?
SHADE: Pazopanib. For an older, favorable-risk patient, I feel like it is worth a shot. If they do not respond or do not tolerate it, then I have no problem switching; but I think it has a role for favorable [older] patients.
KUZEL: I agree, I think there is a role. Observation, for a favorable older patient, may be the best choice.

KUZEL: Do you do your own toxicity management? Do you have dedicated nurse practitioners or physicians assistants who get all those phone calls? SIDDIQUI: I think it is a bit of both; myself and my nurse practitioner.
KUZEL: OK, so it is a combination. If [patients] develop autoimmune toxicities with the combinations, do you stop everything? Do you stop just the ICI, ipilimumab, nivolumab, both? Or do you have more issues with the TKIs?
CONTE: If somebody has diarrhea, it is hard to say. Is it the TKI or is it the pembrolizumab? There are some tricks of the trade; I guess one always assumes it is the TKI, but you do not want to miss colitis. So it’s a little tricky.
KUZEL: If you stop both and they get better, do you restart the ICI ever, and say, “We’ll keep our fingers crossed,” or you are done with both?
CONTE: No, I will try to go in with one, and then go in with the other one.
KUZEL: These have been around long enough that I think [clinicians] feel pretty comfortable with those kinds of strategies at this point, fortunately. Financial toxicity—does anybody have absolutely no luck whatsoever in trying to access any of these drugs for patients? Or [do you] manage to get it?
MULHERIN: It can take a little while, sometimes, to get through the system, but we usually can figure a way. We find a way.
KUZEL: I know some of these come with a 2-week starter pack. Anybody use those, or by the time everything gets going it is 2 weeks anyway? I think lenvatinib has a doseexchange program. So if you start one and you need to replace a dose, the patient is not stuck with 30 days of 20-mg tablets or something, which is nice. Has anybody used any of those? Or do you just do what it takes [to get the therapy]?
MULHERIN: Yes, but again it usually takes a couple of weeks, anyway, to get through insurance.
REFERENCES
1. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530-2540. doi:10.1200/ JCO.1999.17.8.2530
2. IMDC. International mRCC Database Consortium. Accessed July 14, 2022. https://bit.ly/3OCxh0E
3. Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ-specific target lesion assessments with two-year follow-up in CheckMate 9ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. J Clin Oncol. 2022;40(suppl 6):350. doi:10.1200/JCO.2022.40.6_suppl.350
4. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen