Roundtable Discussion: Panelists Debate Therapy Options for R/R Multiple Myeloma With Renal Impairment
During a Targeted Oncology case-based roundtable event, Saad Z. Usmani, MD, MBC, discussed the case of a patient who progressed on first- and second-line therapy for multiple myeloma and did not receive a stem cell transplant.
Saad Z. Usmani, MD, MBC (Moderator)
Chief, Myeloma Service
Memorial Sloan Kettering Cancer Center
New York, NY


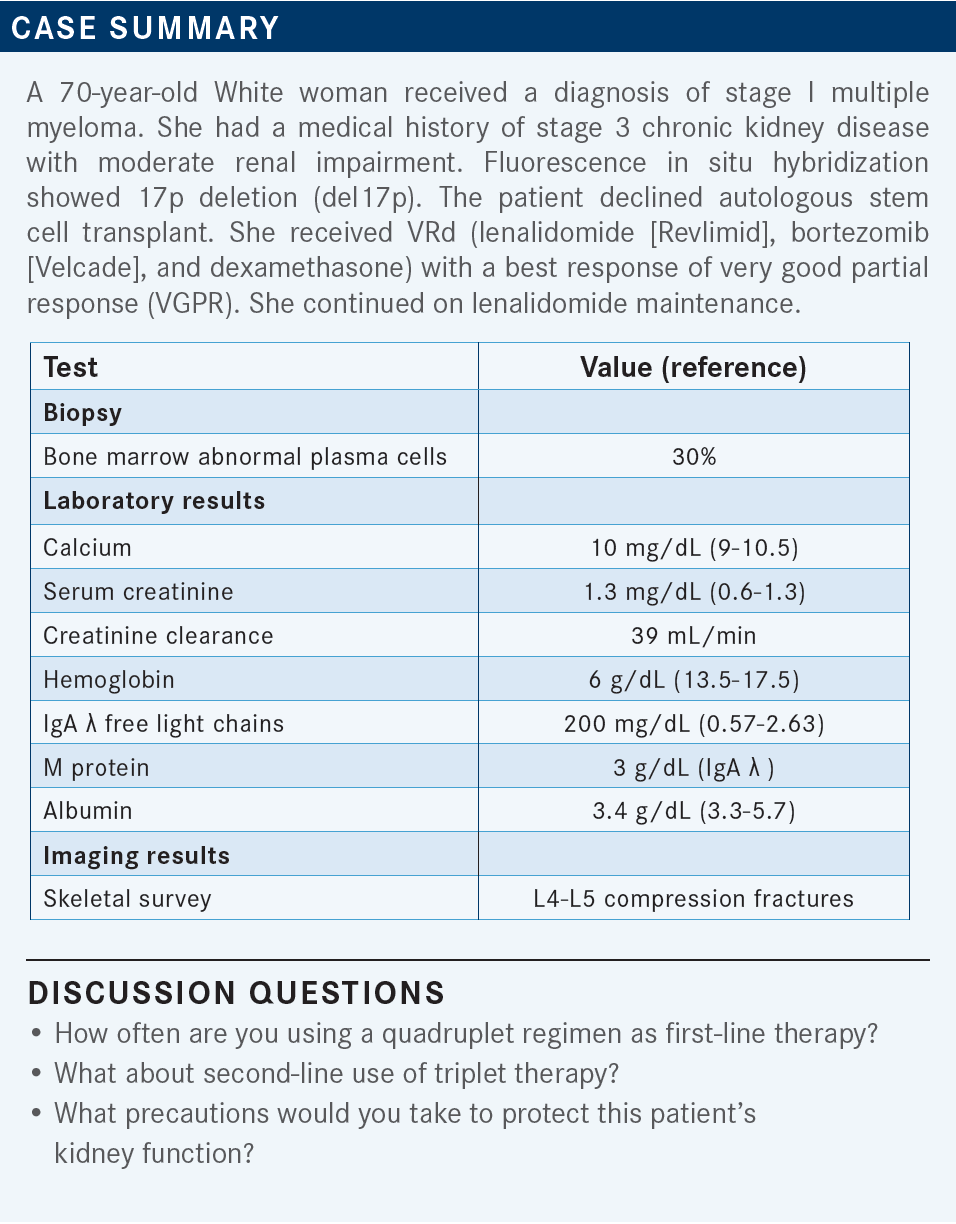
USMANI: This patient [started treatment in 2019]; now we are in 2022, and our perceptions about the data have changed. How many of you are using quadruplet regimens as part of the treatment?
STEINBERG: I would say over the past year or so I’m starting to use quadruplets, maybe in the past 8 to 10 months.
ROTKOWITZ: I think the data from trials like GRIFFIN [NCT02874742]1,2 and CASSIOPEIA [NCT02541383]3 are very impressive, but the difficulty in the community is, I’ll reach out to local and regional thought leaders, and everybody tells me something different. Someone will say, “I’m always using quadruplets,” someone will say, “I’m never using quadruplets,” and I think there may be an additive fashion, where I’ll use VRd and see them not responding and then add in the anti-CD38 agent. For some patients who I think are high risk up front, I feel that the quadruplet makes a lot of sense.
[For second-line therapy], I’m using triplets across the board. I think everyone is triple-class refractory by third line. But when I move into the second line, if they [previously received] VRd, I’m probably using DRd [daratumumab (Darzalex), lenalidomide, and dexamethasone], DVd [daratumumab, bortezomib, and dexamethasone], or KPd [carfilzomib (Kyprolis), pomalidomide (Pomalyst), and dexamethasone] depending on what they got up front. I’m definitely using a triplet or a quadruplet in the first line and definitely a triplet in the second line.
STEINBERG: I’d say there’s no right and no wrong, but a former colleague of mine told me, “Why would we want to leave our best ammunition unused?” We might as well try to use [quadruplet] in the beginning and give it the best shot to start with. That’s when I’m philosophical. I thought it might be a good idea to try it, and I have been for the past several months. I’ve got to worry about toxicity, but it’s been manageable.
USMANI: There has been a lot of discussion around the quadruplet use in the front line, and one point that we try to make in these forums is we’re not using quadruplets forever. It’s for 4 to 6 cycles, then patients go on to their transplants and maintenance. And if they’re relapsing 2, 3, or 4 years later, there’s no reason why you can’t use the bortezomib or the daratumumab with something that they haven’t seen before. It’s all about trying to get deep responses in that first year of diagnosis. Dr Rotkowitz raised an important point about the high-risk patients and weighing the risks and benefits.
GORSHEIN: I have been using it increasingly, as well, in the past 6 to 12 months. For me it’s a more attractive option, potentially, for standard-risk patients, if they’re relatively young and have a good performance status. With high-risk patients, I tend to talk about that vs KRd [carfilzomib, lenalidomide, and dexamethasone], just because of the small sample size in the GRIFFIN [trial] for the [patients with] high risk.1,2
PREET: I usually use quadruplet therapy in high-risk patients, and it also depends on whether I get the insurance approval for the medication; that’s a problem here. Other than that, triplet therapy has worked very well in a lot of patients. The patients who have been newly diagnosed are mostly going on quadruplet therapy, [whereas] the previous ones are on triplet therapy.
CHAWLA: I have used the 4-drug regimen also. I recently had a younger patient in his 40s with high-risk disease, and he had an excellent response to the quadruplet. In the past year I have been using it more and more but for the high-risk younger patients.
USMANI: Would you all agree with [Dr Rotkowitz that] second-line use is mostly triplet regimens? I think that debate is beyond discussion now because we have so many triplets that have demonstrated good efficacy compared with [giving only] 2 drugs.
What would you think about picking one regimen vs the other based on renal function or dose adjusting some of the drugs based on renal function? Do you think about whether the renal function issue is due to a nonmyeloma reason vs due to myeloma?
SHANI: Does the patient have any comorbid conditions [such as] diabetes and hypertension?
USMANI: The patient did have CKD [chronic kidney disease] stage III at baseline. They did have hypertension for the past 15 years. That is another reason for this patient to have the renal insufficiency beyond myeloma. Do you have thoughts about [your choice of] lenalidomide or pomalidomide?
SHANI: I would reduce the lenalidomide, obviously, but [I would] definitely use bortezomib and dexamethasone, and maybe daratumumab.
USMANI: You don’t need to adjust daratumumab for renal insufficiency.
STEINBERG: Maybe daratumumab/CyBorD [cyclophosphamide, bortezomib, and dexamethasone] rather than an IMiD [immunomodulatory drug].
USMANI: I would think about daratumumab/CyBorD. I think it’s a reasonable thing to start patients on, especially if when one feels that the renal function [issue] is primarily because of the myeloma burden, before switching the cyclophosphamide to an IMiD.

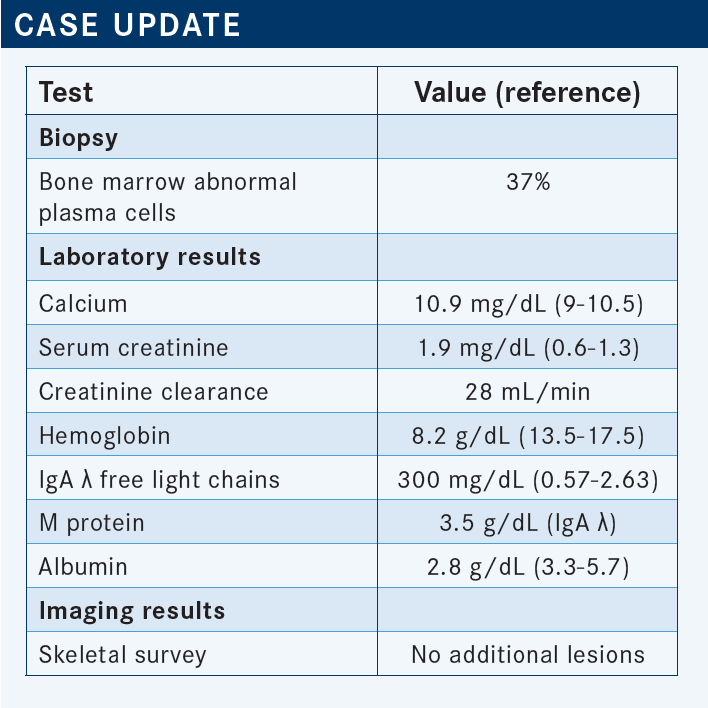
USMANI: We can start discussing this case in a bit more detail. The patient had a relapse after 2 years. She was on lenalidomide maintenance when she was found to have worsening renal function, and the restaging shows 37% bone marrow plasma cytosis now. The M spike and light chains are going up fairly rapidly, so they get worked up.
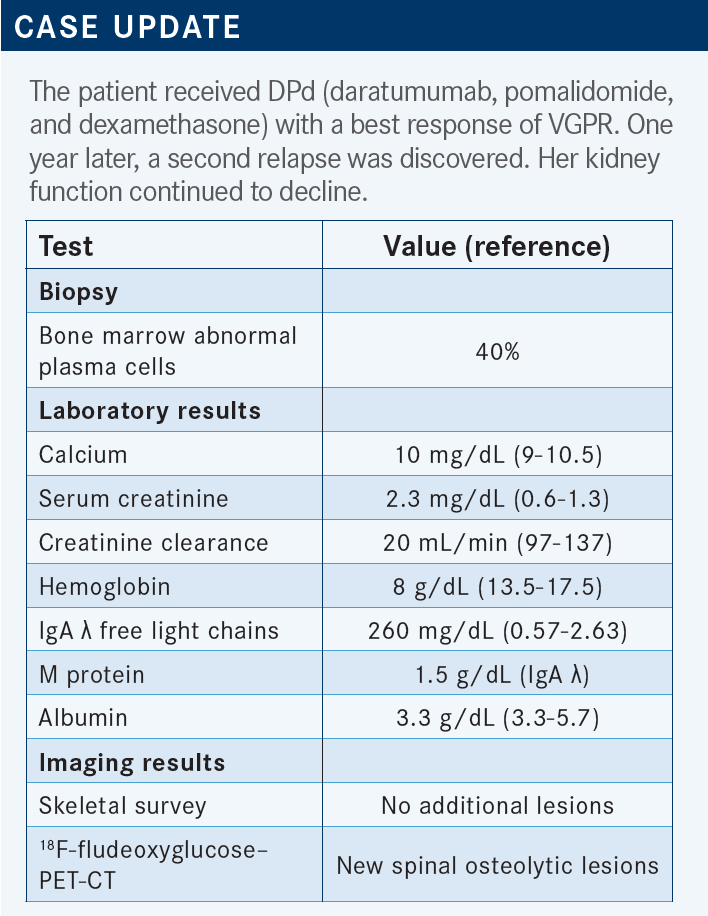
USMANI: They start DPd and then they get to VGPR, and they [maintained their response] for a year before they have another relapse. Now their kidney function is declining further. The serum creatinine level is 2.3 mg/dL, creatinine clearance has declined to 20 mL/min. I think we are going to face some interesting choices for the next line.
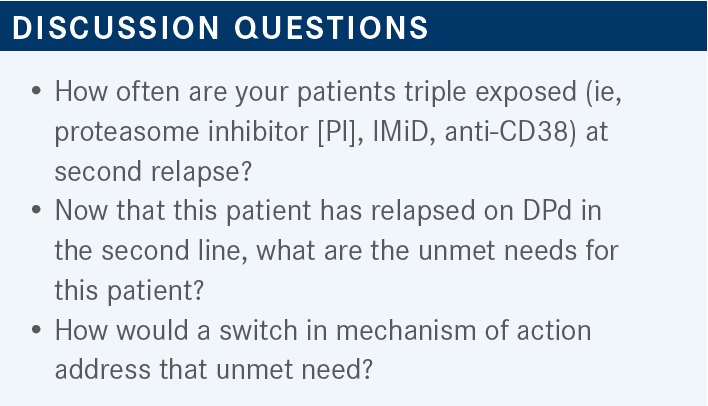
USMANI: This patient is now PI, IMiD, and anti-CD38 exposed after the second relapse, and this patient relapsed on DPd in the second line. Just looking at this case, how often do you see these kinds of patients? And when you do see them, what are your thoughts? What are the areas of unmet need for patients like this? They’re clearly IMiD refractory now because they have progressed on lenalidomide and pomalidomide. They’re anti-CD38 refractory. They’re PI exposed but not refractory.
STRAUSS: I would love to put the third-line patients on CAR [chimeric antigen receptor] T-cell therapy, but it’s been very hard for us to get spots. There’s a huge waiting list. Absent that, other BCMA [B-cell maturation antigen]–targeted therapies like belantamab mafodotin [Blenrep] are a good option. I’ve used selinexor [Xpovio].
PREET: I had one patient who progressed on multiple regimens, and she was in very poor health. I used bortezomib and cyclophosphamide, and somehow the cyclophosphamide did help us a lot. She was in a nursing home and was not ready to pay for expensive medications.
USMANI: That’s doable. If they haven’t seen bortezomib in a while and they haven’t seen an alkylator in a while since they got melphalan [Alkeran], CyBorD would be a reasonable choice for this kind of a patient.
ROTKOWITZ: I’d love to send these patients for idecabtagene vicleucel [ide-cel; Abecma] or ciltacabtagene autoleucel [cilta-cel; Carvykti], but there are no spots and no availability. There are local/regional clinical trials with teclistamab or talquetamab with a bispecific antibody, which I think would be great for these patients. My No. 1 rule is don’t volunteer for anything. Don’t get involved in things that are going to make your life more difficult, and it’s very difficult to get belantamab in the community.
The ophthalmologic necessity for follow-up is very difficult to try to maintain. So if the patients are not candidates for a bispecific antibody, then you start looking at other agents for multiple-relapsed disease: ixazomib [Ninlaro], elotuzumab [Empliciti], and selinexor a bit later down the line. Or you try to recycle something.
I tend not to use another CD38 after daratumumab, so I wouldn’t use isatuximab [Sarclisa]. The patient already got carfilzomib, so you’re pretty limited if the patient can’t go for a clinical trial, but you may try to look at other novel recycling of some other kind of combination that’s there.
USMANI: Has anyone treated patients with belantamab mafodotin in their clinical practices, and what have been their experiences?
STEINBERG: I gave it to one patient who had received 7 prior regimens, and the medication was just approved, and she responded to it. I was really happy. The problem was, it was a heroic effort just to try to get it started. We had to find an ophthalmologist who felt they could be onboard with this, and then apply for the REMS [Risk Evaluation and Mitigation Strategy]. I had to enroll her in the REMS on her [behalf]. It was very frustrating. Then we had to get the ophthalmologist’s [document], then upload it. It was very frustrating, but the patient did respond.
USMANI: That’s good feedback. The theme that I’m hearing is we need more mechanisms of actions for those kinds of patients. Our options become limited. And then trying to get them to CAR T-cell therapy or trials [of bispecifics], if possible, is something that many of you would consider; but with CAR T cells, we are stymied because there are only so many slots.
We are hoping some of that will be mitigated now that cilta-cel is approved. So we will have at least twice as many slots; maybe instead of 1 per month, maybe 2 slots per month at each of the transplant centers. This patient gets VRd followed by lenalidomide [maintenance], and then they have their first relapse. They forgo [autologous stem cell] transplant. Then they get DPd after second relapse. And then we talked about all these different options.
This patient is a 73-year-old with worsening renal function now. Beyond the mechanisms-of-action discussion, I think there are so many other [issues] that we can talk about: patient comorbidities, toxicities to prior therapy, performance status. And do you change the mechanism of action when you get to that relapse? Then the co-pays; that’s a challenge. Dr Rotkowitz brought up issues with care coordination with the eye care specialist [when using] belantamab mafodotin.
GORSHEIN: I’ve had 1 patient who had a good response to [belantamab] after 6 or 8 weeks, but the logistics of it and the frequent visits with an ophthalmologist—overall, the treatment and the associated [issues] with it just became too cumbersome for the patient. He chose to go off the therapy but had a partial response to treatment after about 2 or 3 cycles.
USMANI: The thing with belantamab, if someone is going to respond or have eye issues, you’ll know within the first 2 doses. If they’re not having any response after 2 doses, the likelihood of getting a response is low. If they’re not having eye symptoms after 2 cycles, it’s likely they won’t get them. If you’re going to go that route, it’s a lot of work on the front end.
What I’ve done with my patients is give those first 2 cycles to see what’s happening. If they’re responding [and not having] eye symptoms, I keep on going. If they’re responding, with eye symptoms, I back off and let them rest. We’ll come back a few weeks later with dose attenuation. If they haven’t had a response and have eye symptoms, there’s no point continuing belantamab mafodotin. That’s how I approach that. Right now, we don’t have any bispecifics that are FDA approved, so outside clinical trials if patients are running out of mechanisms of action, I would think about belantamab. I agree, it’s a logistical challenge, trying to get that going.

USMANI: This patient—[because she] had worsening renal impairment and del(17p) and based on prior therapy—was XVd. She had bortezomib exposure but was not refractory, and she hadn’t received a PI in a while. She is being given weekly selinexor along with it, and she got to a VGPR.

STRAUSS: I used it once in combination with carfilzomib and dexamethasone, and [the patient had] a very bad complication from the combination. I think he had a TMA [thrombotic microangiopathy] from the carfilzomib. He recovered, fortunately, and then switched to XVd, and he did OK with it. He tolerated it but then progressed after 6 months or so. This was third line.
GORSHEIN: I’ve used it also, with pomalidomide and carfilzomib, fourth line and fifth line. I used selinexor, pomalidomide, and dexamethasone [XPd] when the patient wanted to maintain their quality of life. They like the all-oral option, as well, with the weekly dosing of selinexor, and they’ve been on it now for 3 or 4 months. So far, they’ve had a partial response and are tolerating it relatively well.

STEINBERG: I had worked at a place where it was given a lot, and though I didn’t personally initiate it, I saw a lot of patients who were on it. I have a lot of experience in that regard, and GI [gastrointestinal] toxicity was one of the big things I would caution the patients about [From the Data4]. Be prepared to have antidiarrheals available. That was the biggest take-home I got from my experience with the drug.

SHANI: Dr Steinberg, why weren’t you initiating it?
STEINBERG: Part of it was…I preferred other medications that I felt more comfortable with. For example, we haven’t discussed carfilzomib. This patient wasn’t getting carfilzomib. I probably would have dipped into that well instead. I just felt more comfort with that. The other thing is with an oral drug, sometimes they’re hard to get and you get these gigantic co-pays that patients have to pay. There’s the financial toxicity I would warn the patient about. For those 2 reasons. That’s why, for example, with the belantamab patient, in many ways I preferred the IV option as opposed to oral.
SHANI: You never know whether they are taking it, especially when there was severe nausea and vomiting.
ROTKOWITZ: I would just add that antiemetics are really important. You need to have those onboard. I think [you need] diarrheal prophylaxis and then meticulous cytopenia management. Dose modifications are sometimes warranted to keep the patient on the drug, and you need to monitor their blood counts closely and make sure the nurse practitioners are following up with them very closely.
USMANI: Would you do that early on as you’re starting treatment and then back off from that more intensive approach?
ROTKOWITZ: Absolutely. I do the same in diffuse large B-cell lymphoma, as well, for the patients after multiple lines of therapy. I think the toxicities tend to come earlier, rather than cumulatively, for this drug. I think if the patients are tolerating [the treatment] well, I feel comfortable backing off a little bit.
CHEUNG: My biggest concern for this patient is the renal disease. With selinexor, [there will be] nausea, vomiting, diarrhea; it is a setup for disaster. You need a patient to adhere [to not only treatment] but also follow-up and reporting, and be able to maintain hydration.
USMANI: Yes. Typically, in our practice, we would have patients come in once a week for possible IV hydration during that first cycle of treatment, for more effective nausea management and management of cytopenias. Depending on how they handle it during that first cycle, we’d continue the same during the second cycle. Typically, you come to some sort of a reconciliation of symptoms with dose management or dose adjustments by the end of the second cycle, and you’ll know whether the patient is responding. I agree with you. I think paying attention to hydration status during that first cycle in someone with renal impairment is important.
CHEUNG: I think selinexor is a great drug. I’m shy of using it because of the GI toxicity. For this patient, I would use a cyclophosphamide/IO [immuno-oncology] combination before I would use selinexor because it is IV, and also, I don’t worry much about renal function with it.
USMANI: I think Dr Steinberg brought up the same point about some alternative options [because] carfilzomib hasn’t been used for this patient. Neither has an alkylator.

USMANI: Looking at how the landscape for first-line treatment is evolving, where do you feel selinexor will get used mostly, looking at the various combinations in the STOMP study [NCT02343042]?5-7
GORUSU: Maybe XPd. Because practically speaking, if we are talking about in the third-or-so line, we must have likely used the PI, and daratumumab must have already been used. Somehow, a little bit, trying to be careful with carfilzomib. If I were to pick, I think I would go within the XPd space.
USMANI: That makes sense. It’s an all-oral option. I think that’s very reasonable.
STEINBERG: One of the therapies that we haven’t mentioned too much, and you brought it up, was alkylators. Or just giving this patient an IV DCEP [dexamethasone, cyclophosphamide, etoposide, and cisplatin] or VD-PACE [bortezomib, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide], or something [similar].
I don’t think the renal dysfunction would necessarily exclude the patient from getting melphalan or high-dose chemotherapy, to at least to give this patient some breathing space, at least temporarily, and then put them on some kind of maintenance. Then it’s time to think about what you want to give them.
USMANI: I think that’s a good point. The patient is essentially alkylator naïve, so you can think about employing some old-fashioned chemotherapy, especially if they’re presenting with high-burden disease and you can modify doses of treatments based on renal function as well.
REFERENCES
1. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/ blood.2020005288
2. Kaufman JL, Laubach JP, Sborov D, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of GRIFFIN after 12 months of maintenance therapy. Blood. 2020;136(suppl 1):45-46. doi:10.1182/ blood-2020-137109
3. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. doi:10.1016/ S0140-6736(19)31240-1
4. Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563-1573. doi:10.1016/ S0140-6736(20)32292-3
5. White DJ, Chen CI, Baljevic M, et al. Once weekly oral selinexor, pomalidomide, and dexamethasone in relapsed refractory multiple myeloma. Blood. 2021;138(suppl 1):2748. doi:10.1182/blood-2021-148759
6. Lentzsch S, Lipe B, Tuchman SA, et al. Efficacy and safety of selinexor-containing regimens in patients with multiple myeloma previously treated with anti-cd38 monoclonal antibodies (αCD38 mAb). Blood. 2021;138(suppl 1):1651. doi:10.1182/blood-2021-150232
7. Mo CC, Jagannath S, Chari A, et al. Selinexor for the treatment of patients with previously treated multiple myeloma. Exp Rev Hematol. 2021;14(8):697-706. doi: 10.1080/17474086.2021.1923473

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More