Gasparetto Discusses Sequencing Selinexor in Relapsed/Refractory Multiple Myeloma
During a Targeted Oncology case-based roundtable event, Cristina Gasparetto, MD, discussed data supporting the use of selinxor for patients with relapsed/refractory multiple myeloma.
Targeted OncologyTM: What data could inform the decision of whether to treat a patient such as this with selinexor (Xpovio), bortezomib, and dexamethasone?
Cristina Gasparetto, MD
Cellular Therapy Specialist
Hematologic Oncologist
Stem Cell Transplant Specialist
Duke University
Durham, NC

GASPARETTO: The phase 3 BOSTON study [NCT03110562] investigated this combination. The results of this study led to FDA approval for the use of this combination at first relapse.1 There were 402 patients enrolled and randomly assigned on the study. Patients in the experimental arm received 100 mg of selinexor once a week; bortezomib, which was also given once a week; and dexamethasone, which was given on the same day as bortezomib and on the day after bortezomib. In the control arm, patients were treated with bortezomib plus dexamethasone, but the bortezomib was given [according to the standard twice-weekly regimen], on days 1, 4, 8, and 11.2
When the study was designed, it was planned that the patients in the experimental arm would receive a 40% lower bortezomib [dose] and a 25% lower dexamethasone [dose] than the patients in the control arm. For the first 8 cycles, patients in the control arm received bortezomib twice a week and then, starting with cycle 9, these patients changed to weekly administration of bortezomib. Also at cycle 9, the dexamethasone administration was reduced from 4 times weekly to twice weekly.
In the experimental arm, bortezomib was given only once weekly [throughout the study], and dexamethasone was given twice weekly throughout the study. The primary end point was progression-free survival [PFS], but there were also important key secondary end points: overall response rate [ORR], depth of response, and the [rate of] peripheral neuropathy of grade 2 or more.
Other secondary end points were overall survival [OS], duration of response [DOR], time to next therapy, and safety. The patients were stratified based on prior therapy, the number of prior lines, and whether they had received a proteasome inhibitor [PI], and on the stage at study entry determined according to the Revised International Staging System.2
What were the baseline patient characteristics of this study?
The number of patients with high-risk cytogenetics was quite high, about 50% of the population, because patients with a 1q21 amplification were included, [as were patients with] del(17p), t(14;16), and t(4;14). The median age was similar between the experimental and control arms at 66 years vs 67 years, respectively. The ECOG performance status was good, between 0 and 1 for most of the patients; about 10% of the patients in each arm had an ECOG performance status of 2.
There was an equal distribution of disease stage at screening between the experimental and control arms [for stage I or II, 89% vs 86%, respectively: for stage III, 6% vs 8%, respectively]. In both arms, the time since diagnosis was [almost] 4 years in both arms. Also in both arms, the percentage of patients who had received 1 prior line of therapy was about 50% and the percentage who had received 2 prior lines was [about 30%].
In the experimental and control arms, the percentage who had received 3 prior lines was 16% and [21%], respectively. Most of the patients [in both arms] had been exposed to a PI, bortezomib, carfilzomib [Kyprolis], or ixazomib [Ninlaro]. Only about 6% of the patients in the experimental arm, vs 3% in the control arm, were refractory to daratumumab. [Other possible prior therapies were] the immunomodulatory imide drugs [IMiDs] lenalidomide and pomalidomide [Pomalyst].2
What did the data reveal about the efficacy of this regimen in the overall population and in various subgroups?
The primary end point of the study, PFS, was met. The median PFS for the experimental arm was [13.93 months; 95% CI, 11.73-not evaluable] vs [9.46 months; 95% CI, 8.1-10.78] for the control arm [HR, 0.70; 95% CI, 0.53-0.93; P = .0075].2
In this study, the selinexor combination benefited patients across subgroups.2 Subgroup analyses are important to me when I review data because I’m [considering whether the experimental] therapy is appropriate for my particular patients, given their age, performance status, and other factors. An important [factor in the patient population of this study is] prior exposure. Remember, the percentage of patients who had received daratumumab was small,2 but these are difficult patients to treat. Before the introduction of these new drugs, the projected OS of daratumumab-refractory patients was less than 8 months.
Now, with the addition of this new therapy, we can [extend] the life of daratumumab-refractory patients. [In the BOSTON study, such patients demonstrated a HR of 0.49 [95% CI, 0.13-1.84]. Among the subgroups defined by cytogenetic abnormalities, patients with del(17p) also performed well [HR, 0.38; 95% CI, 0.16-0.86]. Patients with t(4;14) [also experienced a benefit, though it was a] little less [HR, 0.66; 95% CI, 0.31-1.43]. Additionally, there was a benefit for patients with the 1q21 amplification, which is becoming an important genetic [biomarker], and also a benefit for patients with moderate renal insufficiency, ie, an estimated glomerular filtration rate between 30 and 60 mL/min.
Finally, benefit was observed among all subgroups defined by the number of prior lines of therapy and by the presence or absence of prior stem cell transplant.2 The ORR in the experimental arm, featuring selinexor, dexamethasone, and once-weekly bortezomib, was [76.4%]. In contrast, the ORR in the control arm, featuring only dexamethasone plus bortezomib, which was initially given twice weekly, was [62.3%].
The depth of response was a little better in the experimental arm than in the control arm, with 44.6% vs 32.4% of patients, respectively, demonstrating a very good partial response [VGPR] or better. Patients in the experimental arm also responded faster than those in the control arm, with a median time to response of 1.1 months vs 1.4 months, respectively. The median DOR was 20.3 months vs 12.9 months, respectively, [demonstrating a] definite impact [of the experimental regimen] on the DOR.2,3
A prespecified analysis was performed to compare the outcomes of standard-risk patients with those of patients with high-risk cytogenetic abnormalities. For the high-risk patients, median OS was [22.87] months in the experimental arm vs [24.84] months in the control arm [HR, 0.87; 95% CI, 0.52-1.46; P = .304]; median OS was not reached [NR] for either arm in the standard-risk cohort [HR, 0.75; 95% CI, 0.46- 1.23; P = .129].
The ORRs were similar between the high-risk and standard-risk cohorts [for a given regimen], as were the percentage of patients achieving VGPR or better and the percentage of patients achieving CR. The median DOR [with the experimental regimen] was 13 months for the high-risk group and NR for the standard-risk group, and the time to next therapy was [14 months vs 18 months, respectively].4 These are well-respected data, and I think the outcome was impressive, even in patients with del(17p).
Another important analysis [was performed to investigate the outcomes for] patients with renal impairment. Among patients treated with the experimental regimen, median PFS was [16.62] months for patients with moderate renal insufficiency and [13.24] months for patients with normal renal function.
[Additionally,] the ORRs and depths of response were similar [between these groups], so the combination was tolerated. Now, in patients with severe renal impairment, the [rates of PFS, OR, and VGPR] were lower, of course, but the median DOR was 20.27 months vs 15.34 months for patients with normal renal function and NR for patients with moderate renal insufficiency.5
What did the BOSTON trial reveal about the toxicity profile of selinexor?
In the BOSTON study, in which selinexor was administered once a week, the adverse events [AEs] were like those in the initial STORM study [NCT02336815], wherein selinexor was [administered twice a week in combination with] dexamethasone.
[The AEs in the BOSTON study were] mitigated by the fact that the selinexor was given only once a week. In fact, all the nonhematologic AEs, [most notably] nausea and fatigue, which are common with this drug and affected most of the patients in this trial, were limited to grades 1 and 2 for most patients [in the experimental arm]. Fatigue and nausea of grade 3 or 4 affected only 13% and 8% of these patients, respectively. [Of course], myelosuppression [is to be expected], and [indeed], thrombocytopenia was observed in 60% of the patients in the experimental arm, with [39%] developing thrombocytopenia of grade 3 or 4.
This was higher than what was observed in the control group [27% for all grades, 17% for grade 3 or 4]. Additionally, anemia was a bit more pronounced with the addition of selinexor; [anemia of all grades affected 36% of the experimental group and 23% of the control group].
The most common AEs of grades 3 or 4 were thrombocytopenia, anemia, neutropenia, pneumonia, [and] fatigue. Discontinuation due to AEs affected 21% of the patients in the selinexor arm and 16% in the control arm. The most common reasons for discontinuation were peripheral neuropathy, fatigue, nausea, vomiting, decreased appetite, and thrombocytopenia. Finally, dose modification was common in this trial, with 89% of the patients on the selinexor arm receiving dose modification and [most showing subsequent] improvement of the AEs.2
How do you counsel patients who will receive selinexor?
With selinexor, I always think about gastrointestinal toxicity, particularly nausea, which can be profound at the beginning [and which can lead to] decreased appetite, weight loss, and hyponatremia, and [I also think about] myelosuppression.6
In the STORM study, which employed a twice-weekly administration, the patients needed 3 antiemetics [as soon as they began treatment] with the experimental regimen. When selinexor was given once a week, the toxicity was mitigated. When we use this reduced regimen, we use 2 antiemetics for about the first month or 2, ondansetron [Zofran] in combination with olanzapine [Lybalvi] at bedtime, but after about the first month, when the patient is tolerating therapy, we make the proper dose adjustment. Then the patient doesn’t need the second antiemetic anymore.
Still, you do need to counsel your patient by discussing the new drug and what to expect. We counsel patients about nutrition and intravenous [IV] fluid support and we monitor their blood count. Because of the myelosuppression, particularly the thrombocytopenia, it is important to monitor the blood count. It is also important to monitor the sodium level. We still see some hyponatremia even with the once-weekly dosing schedule, so monitor electrolytes. Also, monitor the patient’s nutritional status and counsel the patient about caloric intake and hydration. We use a fair amount of IV fluids, particularly in the first month. I always tell patients that at least some of the symptoms are going to be worse for the first month or 2, but when we modify the dose as necessary, they will find that they become adjusted.
How does the dosing schedule of selinexor affect its toxicity profile and efficacy?
[The dosing of selinexor greatly affects the profile of grade 3 or 4 AEs.] In the STORM study, wherein 80 mg of selinexor was given twice a week to heavily pretreated patients, thrombocytopenia of grade 3 or 4 affected [58%] of the patients. In the BOSTON study, wherein 100 mg of selinexor was given once a week, only 39% [of the patients were thus affected]. [High-grade neutropenia] affected 21% and 9% of the patients in the respective studies, and the patients in the BOSTON study also experienced less [high-grade] fatigue and hyponatremia. Importantly, most patients required dose reductions from 100 mg to 80 mg or 60 mg [Table2-7].
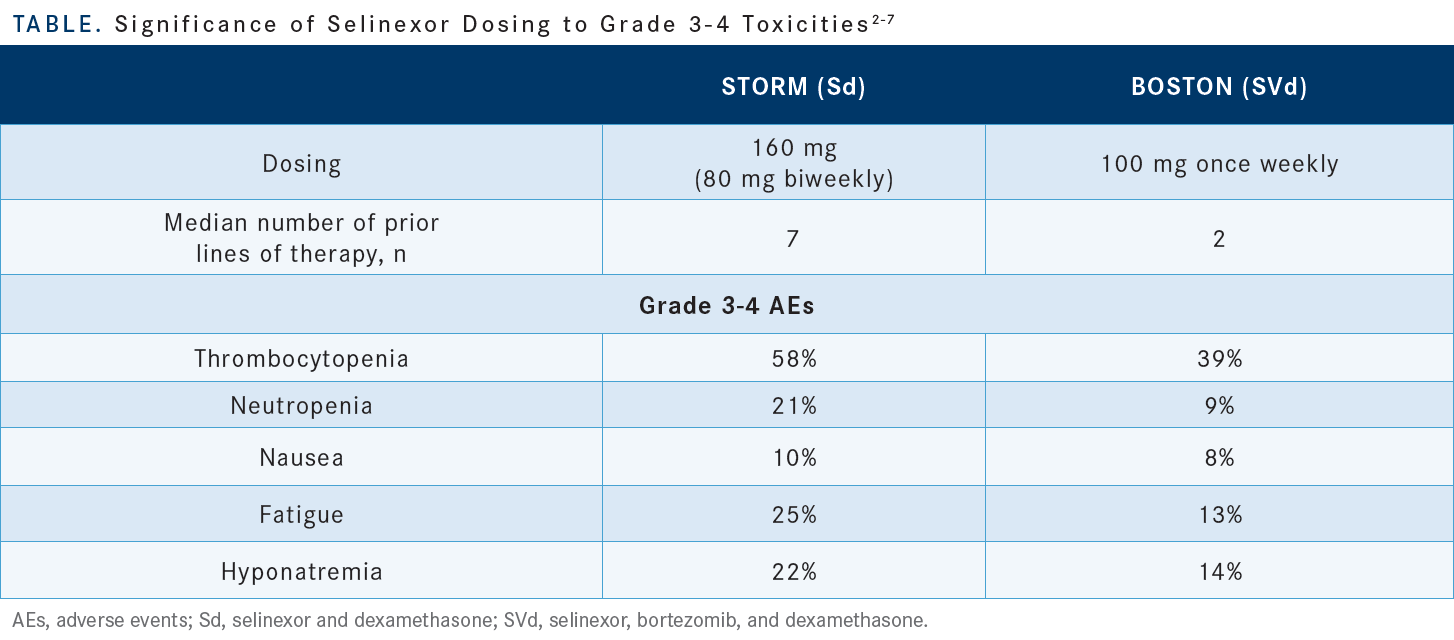
I think you will see, when you start to treat more patients with this combination, that you reach a dosage that becomes the most used dose, and in my clinic that dose is about 60 mg. [Some interesting data show that] the patients who required selinexor dose reduction had a longer PFS than did the patients who were maintained on 100 mg without dose reduction [16.6 months vs 9.2 months, respectively (HR, 0.5678; 95% CI, 0.3614-0.8919; P = .0065)]. Also, the ORR was better for patients with dose reduction than for patients without dose reduction [81.7% vs 66.7%, respectively].8
The message here was simply that the patients were able to tolerate selinexor at the lower dose, adjust to that dosage, and stay on therapy longer. That translates to a better, deeper response and longer PFS. So the recommended starting dose is 100 mg.6 I tell my patients that is a loading dose for the first month and [that we will make] adjustments based on their AEs and [thus be] able to sustain therapy for several months [or possibly even for] a few years.
What data support the use of selinexor in combination with drugs other than bortezomib and dexamethasone?
We can use selinexor in different combinations on the basis of the data from the STOMP trial [NCT01558427]. This was a phase 1b/2 trial in which selinexor was used in combination with different backbone treatments for myeloma (lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab). Patients were assigned to a particular combination based on their prior lines of therapy. It became clear from the beginning that once-weekly administration of selinexor was sufficient.
The dosage was a little lower [when selinexor was used] with the IMiDs because there is more myelosuppression; the selinexor dose was 60 mg when it was used in combination with lenalidomide, 60 or 80 mg in combination with pomalidomide, 80 [or 100 mg] with carfilzomib, and [100 mg] with bortezomib and with daratumumab.
This was important because we were able, in the phase 1 portion of the study, to establish the recommended dose for phase 2, and then [we could] expand the study, enrolling more patients. The combination of selinexor plus carfilzomib and dexamethasone produced an ORR of 78%, and about one-third of the patients achieved a VGPR.9,10 Many patients who received this combination had previously received an anti-CD38 antibody and some were refractory, yet the ORR in this subpopulation was 65% and the PFS was 15 months.11
REFERENCES
1. FDA approves selinexor for refractory or relapsed multiple myeloma. FDA. December 18, 2020. Accessed August 1, 2022. https://bit.ly/3PA67Ij
2. Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563-1573. doi:10.1016/S0140-6736(20)32292-3
3. Dimopoulos MA, Delimpasi S, Simonova M, et al. Weekly selinexor, bortezomib, and dexamethasone (SVd) vs twice weekly bortezomib and dexamethasone (Vd) in patients with multiple myeloma (MM) after one to three prior therapies: initial results of the phase III BOSTON study. J Clin Oncol. 2020;38(suppl 15):8501. doi:10.1200/JCO.2020.38.15_suppl.8501
4. Richard S, Chari A, Delimpasi S, et al. Selinexor, bortezomib, and dexamethasone versus bortezomib and dexamethasone in previously treated multiple myeloma: outcomes by cytogenetic risk. Am J Hematol. 2021;96(9):1120-1130. doi:10.1002/ajh.26261
5. Delimpasi S, Mateos MV, Auner HW, et al. Efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in comparison with standard twice-weekly bortezomib and dexamethasone in previously treated multiple myeloma with renal impairment: subgroup analysis from the BOSTON study. Am J Hematol. 2021;97(3):E83-E86. doi:10.1002/ajh.26434
6. Xpovio. Prescribing information. Karyopharm Therapeutics; 2020. Accessed August 3, 2022. https://bit.ly/3c2DDcu
7. Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. doi:10.1056/NEJMoa1903455
8. Jagannath S, Facon T, Badros A, et al. Clinical outcomes in patients (pts) with dose reduction of selinexor in combination with bortezomib, and dexamethasone (XVd) in previously treated multiple myeloma from the BOSTON study. Blood. 2021;138(suppl 1):3793. doi:10.1182/blood-2021-146003
9. Mo CC, Jagannath S, Chari A, et al. Selinexor for the treatment of patients with previously treated multiple myeloma. Expert Rev Hematol. 2021;14(8):697-706. doi:10.1080/17474086.2021.1923473
10. Gasparetto C, Schiller GJ, Tuchman SA, et al. Once weekly selinexor, carfilzomib and dexamethasone in carfilzomib non-refractory multiple myeloma patients. Br J Cancer. 2022;126(5):718-725. doi:10.1038/s41416-021-01608-2
11. Lentzsch S, Lipe B, Tuchman SA, et al. Efficacy and safety of selinexor-containing regimens in patients with multiple myeloma previously treated with anti-CD38 monoclonal antibodies (αCD38 mAb). Blood. 2021;138(suppl 1):1651. doi:10.1182/blood-2021-150232

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More