Lee Reviews Trials Supporting IO/TKI Combinations in Frontline RCC
During a Targeted Oncology case-based roundtable event, Chung-Han Lee, MD, PhD, discussed the latest data supporting the use of lenvatinib/pembrolizumab as frontline therapy for patients with advanced clear cell renal cell carcinoma.
Chung-Han Lee, MD, PhD
Medical Oncologist
Memorial Sloan Kettering Cancer Center
New York, NY

Targeted OncologyTM: What are the recommended frontline regimens for advanced RCC with clear cell histology?
LEE: The National Comprehensive Cancer Network [NCCN] guidelines have multiple regimens for patients with either intermediate- or poor-risk RCC. They are considered preferred regimens and have category 1 evidence. All these have been randomly assigned against sunitinib [Sutent] as the comparator arm. We have 3 regimens in the tyrosine kinase inhibitor [TKI] plus immunotherapy [IO] category. They include axitinib [Inlyta] plus pembrolizumab [Keytruda], cabozantinib [Cabometyx] plus nivolumab [Opdivo], and lenvatinib [Lenvima] plus pembrolizumab. There is the combination of CTLA-4 and PD-1 inhibitors ipilimumab [Yervoy] plus nivolumab. Also in the preferred category but without category 1 evidence is cabozantinib monotherapy.1
The disclaimer we always give in advance is, you can’t do cross-trial comparisons….All first-line trials for the preferred frontline regimens in RCC had category 1 evidence, and they were all randomly assigned against sunitinib with varying [numbers of patients with] intermediate-, favorable-, and poor-risk disease, but [patients with] intermediate[-risk disease were] the dominant group. All drugs showed a benefit from an overall survival [OS] standpoint, with hazard ratios that were statistically significant. The combination of ipilimumab/nivolumab had an objective response rate [ORR] in the intent-to-treat population of 39%, and the IO/TKI combinations [had an ORR that] ranged from 56% to 71%.
The complete response [CR] rate for ipilimumab/nivolumab was 12%, axitinib/pembrolizumab was 10%, cabozantinib/ nivolumab was 12%, and lenvatinib/pembrolizumab was 16%. The risk of primary progression of disease was 18% for ipilimumab/nivolumab, 11% for axitinib/pembrolizumab, 6% for cabozantinib/nivolumab, and 5% for lenvatinib/pembrolizumab.2-5 In 2021, there were 2 new IO/TKI combinations. Cabozantinib/nivolumab and lenvatinib/pembrolizumab were subsequently approved for treatment of patients with first-line metastatic RCC.6,7

What data support the combination of lenvatinib and pembrolizumab for clear cell RCC?
Lenvatinib/pembrolizumab was FDA approved based on the CLEAR study [NCT02811861], a 3-arm study comparing lenvatinib at 20 mg daily plus pembrolizumab at 200 mg IV [intravenous] every 3 weeks vs lenvatinib at 18 mg daily plus everolimus [Afinitor] at 5 mg daily vs sunitinib at 50 mg 4 weeks on, 2 weeks off.
To be eligible for this clinical trial, one had to have advanced RCC, be treatment naïve, and have a KPS of at least 70%, measurable disease, and adequate organ function. Patients were stratified based on geographic region and by MSKCC [Memorial Sloan Kettering Cancer Center] risk categories. The primary end point of this clinical trial was progression-free survival [PFS]. Secondary end points were OS, ORR, safety, and quality of life, and exploratory end points [were] duration of response and some biomarkers.5 It was a positive study result, and both lenvatinib plus pembrolizumab and lenvatinib plus everolimus ended up showing a statistically significant improvement in PFS, with a hazard ratio for lenvatinib/pembrolizumab of 0.39 [95% CI, 0.32-0.49; P < .001] and an increase in median PFS from 9.2 months on sunitinib to about 24 months on lenvatinib/pembrolizumab.5
The subgroup analyses that were preplanned favored lenvatinib/pembrolizumab over sunitinib, and these included age, sex, geographic location, different risk categories, performance status, the number of sites of disease, and PD-L1 positivity score. The sites of disease also favored the combination, whether patients had bone or liver metastases, prior nephrectomy, and prior sarcomatoid histology.5
The Kaplan-Meier curves for OS showed an early separation at the 26-month median follow-up, with a hazard ratio of 0.66 [95% CI, 0.49-0.88; P = .005], favoring lenvatinib/pembrolizumab with a statistically significant P value. The hazard ratio favored survival whether patients were PD-L1 positive or negative, with the exception of patients in the IMDC [International Metastatic RCC Database Consortium] favorable-risk category.5 With more extended follow-up after 34 months, the median OS was not reached and hazard ratio was 0.72 [95% CI, 0.55-0.93].8
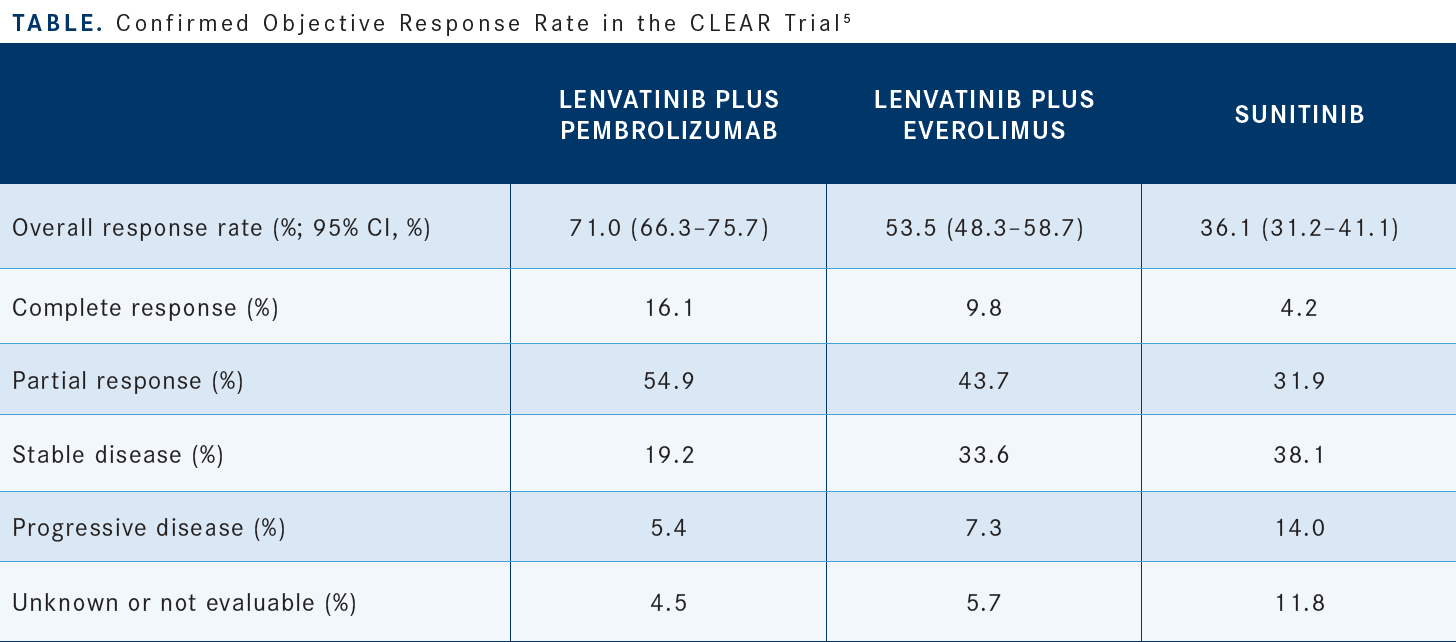
At the early cutoff point, sunitinib had a 36% ORR.5 The combination [of] lenvatinib/pembrolizumab had a 71% ORR, with 16% of patients having a CR compared with 4.2% for sunitinib [Table5]. The primary progression of disease rate for lenvatinib/pembrolizumab is 5.4% compared with 14% for sunitinib. From a safety and tolerability standpoint, as with most TKIs, the percentage of patients who had any treatment-emergent adverse events [AEs] was quite high and as close to 100% as one could get. For severe grade 3 toxicities, we are seeing combination therapy in the 80% range for the different combinations compared with 71% for sunitinib monotherapy.
If you look at the percentage of patients who required some sort of discontinuation of a drug, discontinuation of lenvatinib was about 25% compared with 14% for sunitinib. Discontinuation of pembrolizumab was close to 30%, and discontinuation of both drugs was about 13%. There were higher levels of toxicity with combination therapy, higher levels of diarrhea, a little more hypertension, but relatively low increases in AST [aspartate aminotransferase] and ALT [alanine transaminase] levels and higher levels of proteinuria.
What are the recommended dosage and dose adjustments for lenvatinib plus pembrolizumab?
The recommended dosage for lenvatinib is 20 mg daily, and pembrolizumab is given at 200 mg every 3 weeks for up to 2 years. At the end of 2 years, lenvatinib is continued as a single agent until either progression or toxicity. The lenvatinib is given as both 4-mg and 10-mg pills. The dose reduction scheme starts at 20 mg, goes down to 14 mg, then 10 mg, and then 8 mg.9,10
What data led to the approval of nivolumab plus cabozantinib in the frontline advanced RCC setting?
The CheckMate 9ER study [NCT03141177] led to the regulatory approval of cabozantinib plus nivolumab. This was a randomized phase 3 study comparing nivolumab at 240 mg every 2 weeks with cabozantinib at 40 mg daily. Cabozantinib monotherapy is given at 60 mg, so this is already starting with dose reduction of the cabozantinib. This was compared with sunitinib [at] 50 mg on a 4-weeks-on, 2-weeks-off cycle. Enrolled patients with advanced or metastatic RCC had to have at least a clear cell component but [also] any sort of IMDC risk. The primary end point was PFS.4
The primary end point after a median follow-up of 33 months showed early separation of the Kaplan-Meier curves, with a hazard ratio of 0.51 [95% CI, 0.41-0.64; P < .0001]. The median PFS of cabozantinib/nivolumab was 16.6 months vs 8.3 months for sunitinib [HR, 0.56; 95% CI, 0.46-0.68], which reads as a positive clinical trial result.4,11
[The PFS for] all subgroups favored the combination of cabozantinib/nivolumab, including geographic region, different IMDC risk stratification, PD-L1 positivity or negativity, age, sex, performance status, and whether they had bone metastases or prior nephrectomy.4
The OS, a secondary end point, after a median follow-up of 33 months showed early separation of the Kaplan-Meier curves at the first scans or even before.11 The median OS of the population on cabozantinib/nivolumab was 38 months compared with 34 months on sunitinib [HR, 0.70; 95% CI, 0.55-0.90]. At the 24-month landmark, about 70% of patients treated with cabozantinib/nivolumab were alive compared with 60% for sunitinib.
The ORRs favored the combination, with patients having an ORR of 56% with cabozantinib/nivolumab compared with 27% [with] sunitinib.4,11 The CR rate was 8% with cabozantinib/ nivolumab and about 5% with sunitinib. This didn’t seem to stratify based on whether they were PD-L1 positive or negative. After longer follow-up, the CRs slowly creep as high as 12% for the combination of cabozantinib/nivolumab. For the safety data, in terms of discontinuation of treatment for either drug, about 44% of patients on cabozantinib/nivolumab discontinued: 27% because of disease progression and about 15% related to toxicity.4,11
About 5% or 6% of patients discontinued either cabozantinib alone or nivolumab alone, and about 3% discontinued both at the same time. Most of the AEs like diarrhea, hand-foot syndrome, and hypertension were similar between cabozantinib and sunitinib. One thing they did notice with cabozantinib/nivolumab at a little higher frequency was a risk of increases in AST and ALT levels.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 4.2022. Accessed August 4, 2022. https://bit.ly/3tQpaWN
2. Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085-2097. doi:10.1002/ cncr.34180
3. Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
4. Choueiri TK, Powles T, Burotto M, CheckMate 9ER Investigators, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
5. Motzer R, Alekseev B, Rha SY, CLEAR Trial Investigators, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
6. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. FDA. January 22, 2021. Accessed August 4, 2022. https://bit.ly/3O9DBgt
7. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. August 11, 2021. Accessed August 4, 2022. https://bit.ly/3N7yGLN
8. Choueiri TK, Powles T, Porta C, et al. A phase 3 trial of lenvatinib plus pembrolizumab versus sunitinib as a first-line treatment for patients with advanced renal cell carcinoma: overall survival follow-up analysis (the CLEAR study). Presented at: Kidney Cancer Research Summit 2021; October 7-8, 2021; Philadelphia, PA.
9. Lenvima. Prescribing information. Eisai Inc; 2021. Accessed August 5, 2022. https://bit.ly/3tSHiiB
10. Keytruda. Prescribing information. Merck Sharp & Dohme Corp; 2022. Accessed August 5, 2022. https://bit.ly/39KhOgn
11. Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ-specific target lesion assessments with two-year follow-up in CheckMate 9ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. J Clin Oncol. 2022;40(suppl 6):350. doi:10.1200/ JCO.2022.40.6_suppl.350

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen