Roundtable Discussion: Mikhael Leads Talk on Deep Responses in Transplant-Eligible Multiple Myeloma
During a Targeted Oncology Case-Based Roundtable event, Joseph Mikhael, MD, discusses responses observed in transplant-eligible patients with multiple myeloma.

During a Targeted Oncology Case-Based Roundtable event, Joseph Mikhael, MD, professor, Applied Cancer Research and Drug Discovery Division Translational Genomics Research Institute (TGen), affiliate of City of Hope Cancer Center, discusses responses observed in transplant-eligible patients with multiple myeloma. He was joined in the discussion by Thomas R. Chauncey, MD, PhD, Spencer Shao, MD, Sibel Blau, MD, Jaideep Shenoi, MD, and Andrew J. Cowan, MD.
MIKHAEL: Any thoughts on when a patient asks you, “What’s my life expectancy?” What are you telling them?
CHAUNCEY: [For patients with] middle-of-the-road myeloma—[not high-risk disease]—treatment would typically be an induction and a transplant. Exactly what that prognosis is for someone without any high-risk features, I would defer to you right now, because that median [survival] keeps getting longer and longer….But we have to see how the patient responds, if they get to minimal residual disease [MRD negativity].
MIKHAEL: I completely agree with the approach.
CHAUNCEY: Can you tell me what the latest median is?
MIKHAEL: The bottom line is that, for standard-risk myeloma, the median life expectancy—and you have to remember this is based on the treatments we’ve had up until now, not all the new, promising treatments. We could have a whole other discussion about all the new things that are coming: chimeric antigen receptor T-cell therapy, bispecifics, all that sort of thing. But expected survival for [standard-risk disease] would be at least 8 years, probably closer to 10 years. Now, granted, [for someone under 55 years old], I would hope that it would be longer than that, based on what we’re bringing to them in the future. But that’s the ballpark range we’re looking at.
In the high-risk group, unfortunately, it’s probably half of that. Realistically, our expectation of high risk now is in that 3- to 5-year range, depending on other features. High-risk disease, in and of itself, is a spectrum as well.
It sounded like you were pretty keen on going to transplant. Anyone else have thoughts about transplant eligibility [in patients with multiple myeloma]?
SHAO: [A patient under 55, who] looks healthy, without any organ function problems, and also good functional status [would] be a good candidate for transplant.
MIKHAEL: Agreed. I’m glad you said that, that we don’t just do transplant on age alone. I had the privilege last year of leading the American Society of Clinical Oncology Guidelines panel for myeloma, and this is one of the things that we tried to explicitly say: You cannot base transplant eligibility on age alone. Thinking about organ status and performance status are going to be key. So I think that’s a very reasonable comment.
I tossed in that comment about young patients only because, paradoxically, we talk about all these great survival benefits in myeloma, but when we’re dealing with very young patients, it’s a trickier discussion. I have a patient I’m going to see in clinic next week. When I [performed a] transplant on him just a few years ago, he was 25 years old. So, if I tell a 25-year-old he has a 10-year survival, that’s not that encouraging compared with people who have the median diagnosis of late 60s. It’s, in some ways, a bit of an unmet need when we have the extremely young, the under-40 patients.
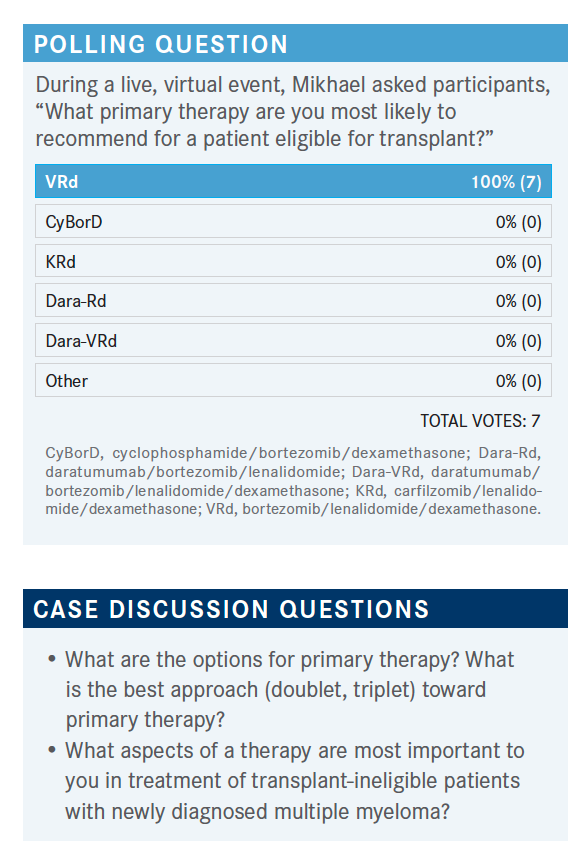
BLAU: I think there is going to be a place for even more triplet regimens, toward achieving MRD negativity, and maybe bypassing transplants. Of course, stem cell collection [will continue to] keep the cells for future with that in mind…. The question [post transplant is] the type of maintenance therapy: single agent, double agent, triple agent—trying to keep the patients in MRD-negative status if possible.
SHENOI: We know from 2017 to 2018 that triplet therapy is better than doublet, so you would not go that backward route. The goal here is to go down to the deepest MRD, but we don’t test it, at least I don’t do it. [In certain situations] I would give at least 4 to 6 cycles and see the trend. If it’s coming down, it’s not mandated to do only 6 cycles. The only issue is that, with more therapy, you can have worse bone marrow suppression. And so the goal is to harvest the cells and do the autologous stem cell transplant subsequently. That’s where you try to tailor [the therapy] to the lowest level, hopefully harvest the cells, and then subsequently order a transplant.
[Patients around] 50 years old have a lot of life left. The important thing is how do I treat—I would not use 4 drugs for [every] patient. I know daratumumab [Darzalex] is the new kid on the block, and people are using it, but I would not use it for [all] patients.
MIKHAEL: What minimum response do you want to see before taking someone to transplant?
COWAN: It’s a great question. First of all, I agree [with the previous comments on] the overall management. But a commonly debated question when we see patients for transplant is: What does the monoclonal protein need to be? Do the free light chains need to be normal? And the answer is always we don’t need to take a one-size-fits-all approach here. If you look at the studies, like the IFM 2009 study [NCT01191060], everyone went to transplant after 4 cycles. If you think of transplant as just another therapy, this is not acute myeloid leukemia where we need to do allogeneic transplants with MRD negativity. The transplant is another line of therapy, and it is often very effective. I would not mandate a minimum monoclonal protein for most patients. But I know that that is sometimes disagreed upon by colleagues.
MIKHAEL: I think it’s very important that we think that way. I think part of it has been because there was this paper that had shown the patients who had the deepest response prior to transplant did better in the long run. But that’s not shocking.
I think the take-home message out of this section is to say that we are looking for deeper and deeper responses. Everybody has mentioned that already, and we are, for the first time in myeloma, really starting to do that. I agree that we are not quite ready to implement MRD negativity into clinical practice, but the concept and the principle of it is the deeper the response, the better. Not that you need that deepest response before transplant, but we are learning that depth, generally speaking, correlates to duration. And so, sometimes I even call it the one-two punch. What you do in your first and your second line of therapy is perhaps more prognostic for a patient than anything else. That’s why we’ve moved from doublets to triplets. I’ll try and make a case in a moment for the fact that we’re probably moving toward, even in the standard-risk setting, a quadruplet-like approach.

MIKHAEL: Do you see that 4-drug combinations are coming? Is there any situation, secondly, in which you would use carfilzomib [Kyprolis] instead of bortezomib [Velcade] as your frontline therapy?
CHAUNCEY: Unless we’re totally surprised, I think whatever end point you pick is probably going to be better [with] the randomized daratumumab plus triplet data. I think that will become the de facto induction.
[Using a] different proteasome inhibitor? I would ask you, in terms of high-risk patients. We just completed a study without high-risk patients showing essential equivalence of carfilzomib and bortezomib—at least that’s my interpretation.1 I think that should have been a wake-up call to not pay attention to comparing across phase 2 studies. I don’t know if there is an ongoing comparator in the high-risk setting; I would ask any of the panel to tell me about it. I think there’s already compendia approval; I think we’re moving toward that. It’s approved in acute myeloid leukemia and I think we’ll probably get there for myeloma. That is going to totally upset what we do downstream.
Then we’re not going to have transplant data, but I think the default is, it’s still the best consolidation we have. Even when you break out the MRD positive and MRD negative, it looks as though—this may not be statistically different—but in the MRD negative, the transplant arm [seems] still superior, although it’s close. I’m not sure the curves are statistically different.
Then we’re not going to have transplant data, but I think the default is, it’s still the best consolidation we have. Even when you break out the MRD positive and MRD negative, it looks as though—this may not be statistically different—but in the MRD negative, the transplant arm [seems] still superior, although it’s close. I’m not sure the curves are statistically different.

MIKHAEL: Do you think we’re going from triplets to quadruplets? And then, secondly, how powerful and useful do you think MRD is?
BLAU: I think there’s no doubt, if we can get these patients into a deeper remission. And I believe we’re going to get away from transplants, my mind always goes there, and also, those patients have horrible adverse and long-term effects from the transplants. I think we’re going to eventually get away from it.
[Also] I think MRD should serve as a surrogate [end point], and we should start using it to understand how we [get better results], because this information is not just going to come from try, fail, try, fail. We have to have a systematic approach to it.
MIKHAEL: I couldn’t agree with you more. We at the International Myeloma Foundation are doing that. We’ve already met with the FDA several times and we’re meeting again with them shortly to make the case that MRD negativity is a surrogate marker of PFS and OS, which would facilitate drug development, but would also answer some of these questions you’ve raised. The myeloma community has tilted much more toward the concept of curing [the disease rather than only controlling the disease]. That’s why I think, with time, MRD negativity will likely be more implemented. I’m a little nervous about making a lot of conclusions based on a patient’s MRD status in clinical practice right now because we don’t know.
We’re starting to learn that MRD negativity might be different in a high-risk patient versus the standard-risk patient, that there’s a difference between MRD once and MRD twice, 6 or 12 months apart. I think it’s going to be one of the tools [physicians use]. You could think of one extreme: You have a standard-risk patient who’s MRD-negative who may be able to de-escalate, if not stop, therapy. By contrast, if you have a high-risk patient who’s still MRD-positive, you might need a whole change of treatment strategy. I think that we’re heading in this direction.
SHENOI: I would agree with that. My only question is: Say you do 4 to 6 cycles, and there’s no MRD, say this patient still has residual disease. [Do you] pursue adding the fourth drug at that time with the other 3? I know there are no data. Or would you plow on [with the triplet]? What would you do if they are MRD-positive and disease is still present?
MIKHAEL: I’d probably still take the patient to transplant and see where they are after it because transplant contributes to depth of response. There’s no perfect number. I like the way Dr Cowan said it: It’s not one-size-fits-all. Most people now, with almost whatever we give them, will at least get a partial response. I’d like to see that before transplant, in general. So with someone like that, I would like to go to transplant.
The bigger question you’re asking, which is what we’re now evaluating in clinical trials, which I think is the most resistant myeloma, is if we give people what we think is the best treatment available and they remain MRD-positive, what do we do with that group? And we’re starting to see that there is a point at which what you’re giving can be given for longer, like we saw in the FORTE study [NCT02203643], there were some people that had their maximal response after 8 cycles, but not very many beyond that. A case could be made that you ask your individual patient how they are doing with the 4 to 6 cycles; is it still going down or has it truly plateaued? And once someone has truly plateaued, we have to think about something different. Is it adding a fourth drug or is it taking a different strategy? We’re now designing studies to do that now that we have a significant fraction of people that go to MRD negativity and a fraction that do not. We can now look at that fraction that do not and randomize them to different strategies. There are a couple of Spanish studies that have taken that exact approach.
COWAN: I think with the MRD assessment—I have been using it routinely in my practice, and it is an academic practice, so I agree that it’s probably not something that we should be doing routinely for everybody. But something I like to emphasize with patients is that the risk assessment is a dynamic assessment. It’s not just where they started; it’s where they are. So if they are MRD-positive today and MRD-positive tomorrow, that probably matters more than if they were high risk and have been MRD-negative for 3 months, or even 6 months. So it is looking where they are right now, and the changes over time that seem to be most important with risk assessment, particularly with respect to MRD.
MIKHAEL: Absolutely. We’re doing a study with our colleagues in Spain showing that the difference between MRD once and MRD twice is really quite considerable, and patients can remain in it. [Is it] worse to have a deep response and lose it, than to have never had a deep response? Bart Barlogie, MD, had shown in Arkansas that highest-risk patients, paradoxically, got a really deep response.2 Many of them even got into complete response. We weren’t [using] MRD back then. But then, they lost [response] quickly. We see this in clinical practice. There’s that patient that gets a deep response, but they relapse within 6 months, or maybe within a year of their transplant. That might be the worst prognostic thing to see in myeloma, that rapid relapse, because unfortunately, the first remission is often the longest remission. So you want to do your best to get someone into a deep and durable initial remission.
BLAU: What about the patients who are high risk and had a deep and durable response, remission, and then relapse with MRD? Are they still in the same category of the patients that relapse within the first year or 2, with a huge spike?
MIKHAEL: The short answer is, they are different. They’re not behaving as much of a high risk; the extremely high-risk patient is the rapid responder. The patient who has high risk and gets into MRD negativity and sustains it for more than a year, prognostically, does better.
References:
1. Kumar SK, Jacobus SJ, Cohen AD. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(10):1317-1330. doi:10.1016/ S1470-2045(20)30452-6
2. Barlogie B, Anaissie E, van Rhee F, et al. The Arkansas approach to therapy of patients with multiple myeloma. Best Pract Res Clin Haematol. 2007;20(4):761- 781. doi:10.1016/j.beha.2007.09.005

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More