Novel Hormonal Agents Show Significant Benefits in Patients With mCRPC
Matthew Smith, MD, PhD, discussed the new hormone therapies entering the treatment landscape for metastatic castration-resistant prostate cancer.
Matthew Smith, MD, PhD

During a virtual Targeted Oncology Case-Based Roundtable event, Matthew Smith, MD, PhD, discussed the new hormone therapies entering the treatment landscape for metastatic castration-resistant prostate cancer.
Targeted OncologyTM: What factors influence your treatment selection?
SMITH: The interesting point here is that the studies [used to discuss this case] all included patients with so-called high risk and PSA doubling times of less than 10 months. The median PSA doubling time in the trials was about 5 months, but the FDA labels [for the approved drugs in this setting] don’t specify doubling time. My understanding of the reason for that is PSA doubling time is not a biomarker itself, it is not a measurement—it is a calculation. I think the FDA was uncomfortable with the idea of using something as unreliable as that calculation because it is very sensitive to the number of values, the interval between values, whether you did the level at the same labs. So, it wouldn’t be inconsistent with the label, but I guess we would say we don’t have as much information about that patient with a longer doubling time.
In the National Comprehensive Cancer Network [NCCN] guidelines, they make the distinction for patients with a shorter doubling time, and they recommend category 1 apalutamide [Erleada], darolutamide [Nubeqa], or enzalutamide [Xtandi].1 I find it a bit curious that they do. Although the preferred recommendation for patients with longer doubling time is observation, they somehow see fit to give other secondary hormone therapies. I think that is a strange recommendation because there is no evidence supporting doing so. We know from other studies, comparative studies, that the newer drugs are superior for things like PSA control or time to progression.
It is a little scary because the list is longer...it may still include drugs like ketoconazole or DES [diethylstilbestrol] therapy that I haven’t used in 10 years because there isn’t much evidence supporting use of those. At least bicalutamide [Casodex] is well tolerated and inexpensive. I certainly can’t say that about some of the other therapies like ketoconazole or DES. They are not expensive but they are fairly toxic. I haven’t written prescriptions for those in a very long time and would not recommend anyone receive them.
I am not supportive of this part of the NCCN recommendations to recommend bicalutamide in the setting for which there is no proof. I would favor including apalutamide, darolutamide, and enzalutamide [rather] than just simply saying we don’t know. But at least the benefit risk may be less favorable, but we at least have established benefit and favorable safety in a higher-risk population.
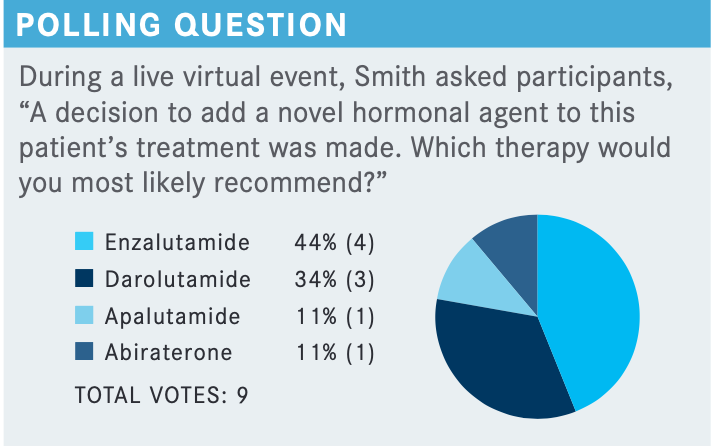
What would you say to oncologists who might be uncomfortable holding off on treatment in favor of observation for a patient with a similar case?
It is a hard sell, [because the argument would be] we don’t have enough drugs that are specifically approved in the setting, and that’s not even factually correct because the label doesn’t exclude those patients. I am not trying to promote off-label or nonevidence-based medicine, but it is a hard thing for a patient to accept. With all these milestones, they have been told, “I am worried your PSA is going up,” and now his PSA is going up, then we just say, “It’s OK, we’ll just watch it.” It is challenging, and I think the field will change as we have more molecular imaging. I suspect those patients who also have detectable disease in places where prostate-specific membrane antigen PET scan has been widely introduced, other parts of the world, it really is a driver for intervention. So it is going to be hard to watch these patients without treatment.

Please discuss the design of the SPARTAN (NCT01946204) trial that led to the FDA approval of the novel agents considered for this patient.
It included about 1200 men with nonmetastatic castration-resistant prostate cancer [CRPC]. I noted shorter PSA doubling times are less than 10 months, [patients were randomized] 2:1 on apalutamide versus placebo, and the primary end point of the study was metastasis-free survival [MFS], with other later end points including overall survival [OS].2
To encourage patients to stay on and do the right thing when they progress to metastatic disease, patients were allowed to then go on to other treatment; in fact, they were encouraged to do so in this trial. The vast majority of patients in the placebo group promptly went on to other potentially life-prolonging therapies such as abiraterone [Zytiga] or the investigator’s drug of choice.
[Most patients on the trial were in their] mid-70s and the study included patients with a PSA doubling time of less than 10 months. Patients were at higher risk with a median doubling time of about 4.5 months.
What were the efficacy results of this study?
There was a big effect on MFS with a hazard ratio of 0.28 [95% CI, 0.23-0.35; P < .001] and a more than 70% reduction in risk of metastasis or death.3 There was also a 2-year delay in development of metastasis in favor of apalutamide with longer follow-up, and the OS became statistically significant.
There you see a 22% reduction in risk of death from any cause with a median survival in the placebo group of [59.9 months and 73.9 months in the apalutamide group (HR, 0.78; 95% CI, 0.64-0.96; P = .0161)].4 The way I think about it is, if you have nonmetastatic CRPC and high-risk features, you have a mortality that is similar to bad de novo metastatic disease. So I think it is one way of quantifying the severity of this condition.
With apalutamide, that’s a little different than the other drugs. There was [a large amount of] rash. Candidly, we treated [a] lot of patients on the trial and a fair number on the commercial product; we don’t see much of it in our patient population for whatever reasons but unmistakably it was an observed event. Usually, we treat patients through it and some patients required topical steroids, but the vast majority of patients were not required for permanent discontinuation.
What was the design and outcome of the PROSPER trial (NCT02003924) that looked at enzalutamide in this patient population?
This was a [similar] study design, a little larger with 1400 patients, 2:1 randomization [on enzalutamide versus placebo plus ADT], a primary end point of MFS, and largely overlapping secondary end points including OS.5 There were nearly identical patient characteristics with patients in their mid-70s, but PSA doubling time in this case was at 3.8 and 3.6 months [in the enzalutamide and placebo groups, respectively]. So, it was a particularly high-risk patient population.
[The primary end point had] nearly identical results to apalutamide, with a significant reduction in MFS of 22 months on enzalutamide [HR 0.29; 95% CI, 0.24-0.35; P < .0001] and a 71% reduction of the risk of death, in this case. The hazard ratio for OS was 0.73 and statistically significant [95% CI, 0.61-0.89; P = .001] after a median follow-up of 48 months. [Median OS on enzalutamide was 67 months versus 56.3 months in the placebo arm].6
Were the safety outcomes of this study similar to patients on apalutamide?
They were very similar to apalutamide, but worth noting is that fatigue was one of the most commonly reported adverse events [AEs], and these are the grade 3 events [in 27 patients].5 Falls and fractures were more common with both enzalutamide and apalutamide [and found in more patients] than patients on placebo.
What trial supports the use of darolutamide in this patient specifically?
In the phase 3 ARAMIS [NCT02200614] study there were 1500 patients but the same patient population and there was 2:1 randomization between darolutamide or placebo. [Primary end points remained MFS with a secondary end point of OS and safety and tolerability.]7
Patients were also in their mid-70s with a median PSA doubling time of about 4.5 months so, again, a particularly high-risk population with about 70% PSA doubling time less than 6 months. There was about a 60% reduction in risk [of] metastasis or death [after a median follow-up of 17.9 months. Median MFS was 40.4 months for patients on darolutamide compared with 18.4 months on placebo].
OS was improved with a hazard ratio of 0.69 [95% CI, 0.53-0.88; P = .003] and that was highly statistically significant like the other 2 trials. [There was also a 31% reduction in the risk of death after a median 29 months of follow-up in this patient group].8
Tolerability of darolutamide appears to be somewhat more favorable than the other 2 drugs, but in all fairness, there has not been a direct head-to-head comparison. This just makes the point that fatigue was also the most commonly reported AE of the more commonly reported AEs [seen in this group], but it really wasn’t much different between darolutamide and placebo—15.8% versus 11.4%, respectively.7 Then falls were the same or less frequent in the darolutamide [arm] than for placebo, and again that’s not even accounting for duration of exposure because patients in their active treatment arm in all 3 trials were uniformly on the study for considerably longer.
When comparing these trials together, which agents and outcomes stand out?
This isn’t a matter of a single study showing a clinical benefit. It is really 3 similar studies that collectively include more than 4000 patients that show this consistent and large benefit in MFS, significant improvement, and then improving many other secondary end points.
Then the drugs were [also] generally well tolerated. Rates of discontinuation [due to AEs] were somewhat higher with apalutamide and enzalutamide compared with placebo. There really was not much difference between darolutamide and placebo. So it was somewhat better tolerated, but I would argue that rates of discontinuation due to AEs for cancer trials were quite low, with only some differences between the drugs that are highlighted. The main differences are less fatigue with darolutamide and no known association with falls or fractures, in contrast to the other 2 agents.9
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 2.2021. Accessed March 30, 2021. https://bit.ly/34xiIXZ
2. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418. doi:10.1056/NEJMoa1715546
3. Small EJ, Saad F, Chowdhury S, et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol. 2019;30(11):1813-1820. doi:10.1093/annonc/mdz397
4. Small EJ, Saad F, Chowdhury S, et al. Final survival results from SPARTAN, a phase III study of apalutamide (APA) versus placebo (PBO) in patients with nonmet-astatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(suppl 15):5516. doi:10.1200/JCO.2020.38.15_suppl.5516
5. Hussain M, Fizazi K, Saad F, et al. PROSPER: A phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). J Clin Oncol. 2018;36(suppl 6):3. doi:10.1200/JCO.2018.36.6_suppl.3
6. Sternberg CN, Fizazi K, Saad F, et al; PROSPER Investigators. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892
7. Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. doi:10.1056/NEJMoa1815671
8. Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi:10.1056/NEJMoa2001342
9. Morgans AK, Shore N, Cope D, et al. Androgen receptor inhibitor treatments: cardiovascular adverse events and comorbidity considerations in patients with non-metastatic prostate cancer. Urol Oncol. 2021;39(1):52-62.doi:10.1016/j.urolonc.2020.08.003

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More