McIver Explores Treatment of a Patient With RET-Mutated Medullary Thyroid Cancer
During a virtual Targeted Oncology Case-Based Roundtable event, Bryan McIver, MD, PhD, discussed the case of a 58-year-old patient with RET-mutant medullary thyroid cancer.

During a virtual Targeted Oncology Case-Based Roundtable event, Bryan McIver, MD, PhD, an endocrine oncologist and deputy physician-in-chief at Moffitt Cancer Center, discussed the case of a 58-year-old patient with RET-mutant medullary thyroid cancer (MTC).
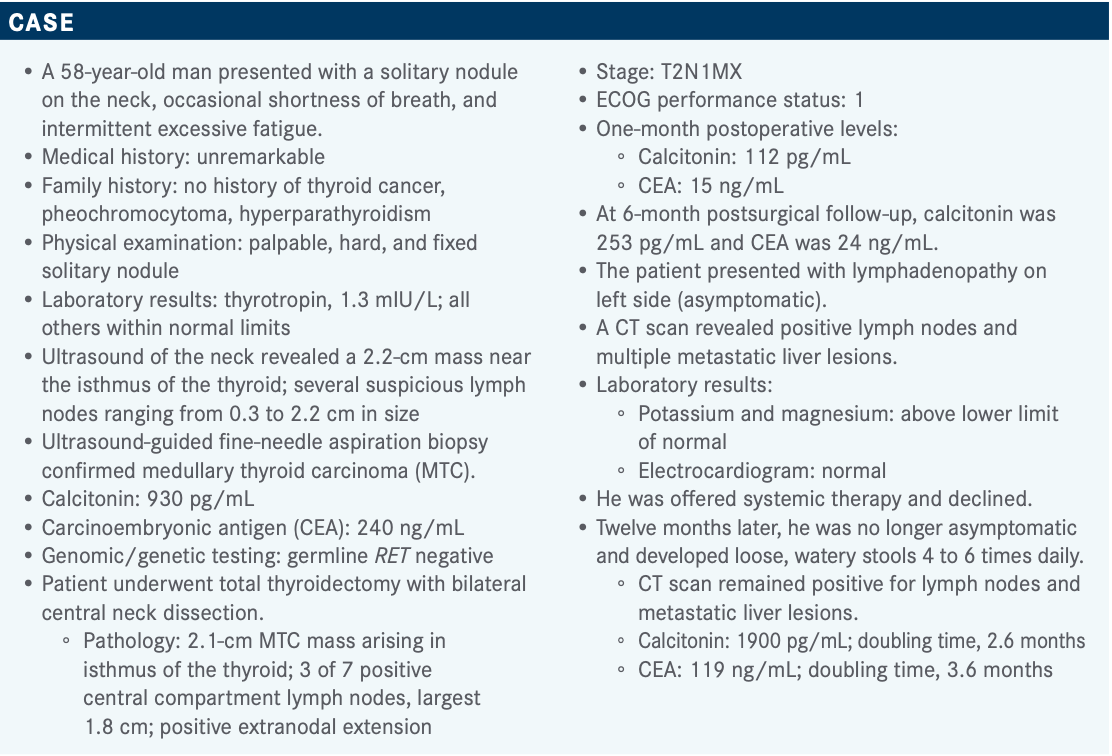
Targeted OncologyTM: What do you think of these poll results?
MCIVER: It’s interesting that among endocrinologists there’s a tendency to use the older drugs, and so I think physicians are going to routinely see patients coming to you who have been exposed to vandetanib [Caprelsa] and cabozantinib [Cabometyx] as first-line therapy.
There’s no question in my mind that the decision to focus on selpercatinib [Retevmo], or arguably pralsetinib [Gavreto], is the appropriate decision. Other than diarrhea, which can be very troublesome in these patients, many patients are remarkably asymptomatic [on these 2 drugs] despite large burden of disease. One of the challenges in initiating systemic therapy in patients with thyroid cancer is that the cancer does not make these patients sick until very advanced stages.
There’s always this reluctance to embark on systemic therapies because these drugs will have adverse effects [AEs]. Thirty percent to 40% of patients started on selpercatinib or pralsetinib will have systemic AEs. That’s something that makes many endocrinologists uncomfortable because they see a patient who is remarkably well and now they’re starting a drug that has significant AEs. One of the things I’ve learned from working with my medical oncology colleagues so closely is that AE management is key—that we can manage the AEs, especially of these targeted agents. What endocrinologists have typically viewed as toxic therapies are remarkably well tolerated so long as we manage the AE profile. I am on board entirely with what all [the participants] suggested, which is that selpercatinib is the drug of first choice in this disease, with pralsetinib as a close runner-up. Vandetanib and cabozantinib are second-line therapies in this disease.
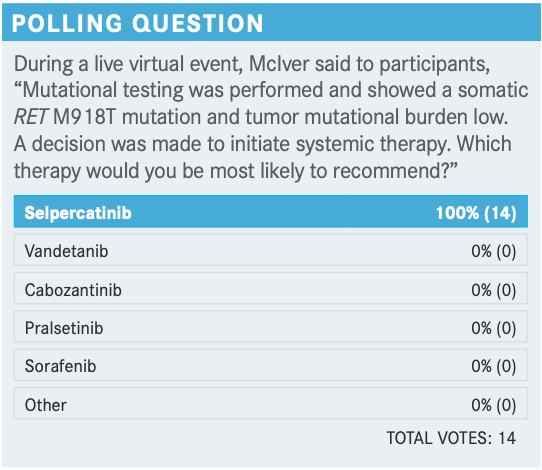
What were your triggers to initiate the systemic therapy? Why would you choose to do it now for this patient?
This patient developed significant systemic symptoms with diarrhea 4 to 6 times a day. This is a patient who has rapidly progressive disease. He has structurally progressive disease affecting the liver in particular but also cervical nodes. This is a patient who clearly [needs] systemic therapies. We cannot manage this by aggressive palliation through surgeries, or radiation therapies, or ablative therapies. This is a clear indication for the systemic therapies.
In my own experience and also based on data at the 2020 European Society for Medical Oncology meeting, there’s clear evidence that selpercatinib and pralsetinib significantly [affect] diarrhea and the paraneoplastic impact of thyroid cancer to improve the quality of life. So I’m glad that everybody is willing to use selpercatinib.
I’ve had several patients who were in both the ARROW [NCT03037385] and LIBRETTO-001 [NCT03157128] studies who had dramatic responses—not just their tumor markers and tumor size but a dramatic impact on the disease. I believe that these drugs are truly game changers in the field of MTC and, to a somewhat lesser extent, the papillary thyroid cancer [PTC] derivatives with RET alterations.
How was LIBRETTO-001 investigated in this setting?
The LIBRETTO-001 data [looked at] selpercatinib in RET-mutant MTC and RET fusion-positive cancer that’s typically poorly differentiated thyroid cancer that is derived from PTC, and also some small number of patients with anaplastic thyroid cancer.1 I will talk about systemic therapy-naïve patients and previously treated patients with each of those types of cancer, medullary and nonmedullary.
In the RET-mutant MTC population, 57% had the M918T mutation. There were a small number of patients with a V804M/L mutation. Extracellular cysteine mutations were present in 19%, and other RET mutations represented 16%. These others were typically insertion or deletion events or combination of deletion and insertion events in these patients. This is an important area for us. We must understand that if all you do is an RET mutational profile, you will miss some of these gene rearrangements and fusion events that occur. Choosing the right test to look for RET abnormalities is important. This needs to be next-generation sequencing. It needs to be DNA- and RNA-based. Typically, the large commercial organizations or academic medical centers that have their own in-house panels will be addressing all these issues.
What was the efficacy for patients in this trial?
The objective response rate [ORR] was remarkably good in RET-mutant MTC that had been previously treated, at 69%. Patients with RET-mutant MTC that had not been previously treated had a 73% ORR. The previously treated RET fusion-positive thyroid cancer population had a 79% ORR. The previously untreated RET fusion-positive thyroid cancer population had a 100% ORR. These are remarkable numbers, the likes of which in thyroid cancer we have never seen.
There were complete responses [CRs] in 12.5% of those with untreated RET fusion-positive thyroid cancer and partial responses [PRs] in 88%. The median duration of response is relatively prolonged, it was not established [in most of the cohorts], but it was more than 19 months in RET-mutant MTC that had been previously treated, 22 months in RET-mutant MTC previously untreated, then 18 months in the previously treated RET fusion-positive thyroid cancer, and uncertain but prolonged in the previously untreated RET fusion-positive thyroid cancer. Thyroid cancer progresses more slowly than non–small cell lung cancer in general, and so the duration of response tends to be more prolonged. Part of this reflects the fact that these cancers are driven by 1 or a small number of mutations. For the most part, they are not polygenic diseases. We do not have high mutational burden. So these cancers are sensitive to the specific mutational driver of RET.
The vast majority of patients had either stable disease or PR with a small number of CRs seen in LIBRETTO-001. These are the patients who have not been exposed to prior tyrosine kinase inhibitors [TKIs].
The durability of that response looked incredibly impressive, and the progression-free survival [PFS] similarly impressive—again, game changing in this disease.
The AEs were similar to what we would expect with this type of drug in general. Dry mouth was common [any grade, 39%]. Hypertension was relatively common but relatively easily managed [30%]. Diarrhea was uncommon [17%]; these patients gain benefit in their pretreatment of diarrheal disease. Fatigue can occur [25%]; send them to your endocrinologist. We’re used to dealing with fatigue all the time in our thyroid cancer patients. There was liver dysfunction because of elevated aspartate aminotransferase level [28%], nausea [15%], and constipation [16%]. In terms of the grade 3 and grade 4 events, they were relatively uncommon [any grade 3/4, 30%].
[This] drug is better tolerated than any of the predecessor drugs. There was certainly some need for dose reduction. About 30% of patients needed a dose reduction [because of treatment-related AEs], but only a very small proportion of patients discontinued the drug [2%]; 2 patients because of elevated alanine aminotransferase level and 2 patients with drug hypersensitivity.
How do you think these data affect practice?
What this emphasizes to me is the need for endocrinologists who care for these patients in the early stages of the disease and medical oncologists beginning to develop a collaboration to ensure [endocrinologists] get access to these patients [at a] point where these patients can benefit from the treatment. One of the challenges I think we have is that endocrinologists are trained in the management of chronic diseases and tend to treat these patients over many years. Our medical oncologists come right at the end of these patients’ lives [and] often are not given the opportunity to see these patients early enough in the disease process. One of the things that I’m keen to do is develop a very strong collaboration between endocrinologists and medical oncologists to facilitate the referral of these patients. The second thing is to train endocrinologists to better understand [the right] time to use these drugs and when it is time to involve our medical oncology colleagues to assist in that process. As far as the data standing out to me, the efficacy data, the duration-of-response data, and the remarkable safety data [with selpercatinib is] truly game changing
Can you discuss the design of the pralsetinib trial?
The ARROW study looked at RET-mutant MTC [that] had been treated with vandetanib or cabozantinib in the past in 67 patients, patients with RET-mutant MTC with no prior systemic therapy in 42 patients, and RET-mutant MTC with prior systemic therapies other than vandetanib and cabozantinib in 10 patients.2
The 2 groups had similar demographics; 59 years was the median age in the previously treated patients and 61 in the treatment naïve [or patients treated with therapies other than vandetanib/cabozantinib]. Roughly 70% of both groups were male. There was a similar ethnic distribution [except for the Asian patients, 5% vs 17%, respectively]. Unfortunately, clinical trials in thyroid cancer, like many other cancers, tend to be heavily weighted toward the White population, which is a disgrace on all our parts. ECOG status was generally very favorable, ECOG 0 or 1 in the vast majority, with only a small number of patients with ECOG 2 status. Central nervous system involvement was around 10%. There were up to 3 prior therapies in 28% of the vandetanib- or cabozantinib-naïve patients. Anti–PD-L1 and PD-1 inhibitors had relatively small numbers. That’s what we would expect because we don’t have compelling evidence of benefit in patients. The majority of the RET mutations were M918 mutations [52%], cysteine-rich domains in 22%, and others including some insertions and deletions.
What was the efficacy of the ARROW study?
The ORR was 60% in the RET-mutant MTC in the vandetanib/cabozantinib groups. In the cabozantinib- and vandetanib-naïve group it was 66%. CR was seen in up to 10% of the previously nonvandetanib/cabozantinib-treated patients, and just under 2% was seen in the previously treated individuals. There was PR in almost two-thirds of patients in each group. The median duration of response was not reached in either of these groups, but greater than 6 months’ response was seen in about 80% [of] these 2 groups, so again very powerful data in favor of [pralsetinib] in MTC.
The AE profile with pralsetinib was a bit different in thyroid cancer compared with lung cancer.3 That’s not surprising, and many of our patients with thyroid cancer are somewhat more sensitive to AEs than the average patients. Musculoskeletal pain reached a grade 3 or 4 in 0.7%, remarkable given that grade 4 is life threatening. I’m not sure what life-threatening musculoskeletal pain involves. Stomatitis, similarly, was 0.7% grade 3 and 4, but grade 1 to 4 in under 20%. Peripheral neuropathy was seen in 20% of patients grade 1 through 4, but none reaching grade 3 or 4. Rash was relatively common but mild [24% any grade]. So this was a well-tolerated drug overall.
How have these data been incorporated into this setting?
The FDA approved pralsetinib for RET-altered thyroid cancers recently.4 In the label you must observe for tumor lysis syndrome.4 The tumor lysis syndrome was reported in patients with MTC receiving pralsetinib. The patients may be at risk of this especially if they have rapidly growing tumors, high tumor burden, renal dysfunction, or dehydration. So good adequate hydration is key in these patients and monitoring these patients with high tumor burden is important.
The data that stand out to me are that this drug is a good alternative to selpercatinib. While I do believe that most folks would use selpercatinib as first line in this disease, I would emphasize we don’t have head-to-head comparison data. I think that’s going to be required. What a great option to have 2 different drugs available for a disease that until recently had very limited treatment options. Efficacy, significant safety, significant tumor lysis syndrome—[this agent is] generally predictable and manageable. I love the fact that we have these drugs now available to us...as we move into this new era of management of thyroid cancer in both medullary and nonmedullary RET mutation and RET rearrangement-driven disease.
Are there other drugs available for patients with MTC?
In contrast with the selpercatinib and pralsetinib data, we do have alternatives, backup plans for use of vandetanib. We have good data that show delay in progression, an ORR of 45% for patients receiving vandetanib versus placebo.5 Vandetanib is available and, at least in my book, remains a good second choice for patients who fail on the selpercatinib or pralsetinib or who have non-RET-driven MTC.
The ZETA trial [NCT00410761] also showed PFS by RET mutation status, demonstrating that the RET-positive disease does respond to the drug....There’s a hint toward benefit for these patients who have RET-positive disease.
AEs were much more common with vandetanib than with pralsetinib and selpercatinib. Lots of patients developed diarrhea [any grade, 56%]; this is a major limiting factor for the use of a drug in a disease where the drug causes diarrhea and the disease causes the diarrhea. So that’s a major issue but also all the usual AEs of TKIs.
In terms of AEs with cabozantinib [in the EXAM trial (NCT00704730)], diarrhea was a major issue.6 These patients had weight loss, loss of appetite, nausea, and fatigue. These drugs are not nearly as well tolerated as the more targeted therapy. So at least in my mind, these drop into the second-line status for these patients.
Dose reductions [with cabozantinib] were very common. Many patients must undergo both dose reduction and ultimately discontinuation of treatment, so quite a contrast from the more targeted therapies
REFERENCES
1. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825-835. doi:10.1056/NEJMoa2005651
2. Hu M, Subbiah V, Wirth LJ, et al. Results from the registrational phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET mutation-positive medullary thyroid cancer (RET+ MTC). Ann Oncol. 2020;31(suppl 4):S1084. doi:10.1016/j.annonc.2020.08.1401
3. Gavreto. Prescribing information. Blueprint Medicines Corp; 2020. Accessed April 2, 2021. https://bit.ly/3kMI8b1
4. FDA approves pralsetinib for RET-altered thyroid cancers. FDA. December 1, 2020. Accessed February 26, 2021. https://bit.ly/2NLNRl4
5. Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134-141. doi:10.1200/JCO.2011.35.5040
6. Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28(11):2813-2819. doi:10.1093/annonc/mdx479

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
Anticipating Novel Options for the RAI-Refractory DTC Armamentarium
May 15th 2023In season 4, episode 6 of Targeted Talks, Warren Swegal, MD, takes a multidisciplinary look at the RAI-refractory differentiated thyroid cancer treatment landscape, including the research behind 2 promising systemic therapy options.
Listen