Moon Explores Use of Avelumab Maintenance in Metastatic Bladder Cancer
Helen Moon, MD, reviewed the data from the JAVELIN Bladder 100 trial, which support the use of avelumab as maintenance therapy for patients with metastatic bladder cancer.
Helen Moon, MD

Helen Moon, MD, hematologist/oncologist, Kaiser Permanente Riverside Medical Center, and principal investigator, Cancer Clinical Trials Access Program at Southern California Permanente Medical Group, reviewed the data from the JAVELIN Bladder 100 trial (NCT02603432), which support the use of avelumab (Bavencio) as maintenance therapy for patients with metastatic bladder cancer.
Targeted OncologyTM: How do you decide whether a patient’s bladder cancer is platinum eligible or not?
MOON: Platinum [eligibility] is probably the first dividing line in bladder cancer. One definition [is from] the Consensus Definition of Bladder Cancer Working Group. Essentially, the group surveyed 56 genitourinary medical oncologists and it was pretty interesting. It was a 2019 abstract, but one would argue that that survey was probably a little bit older. The parameters [were] used to define platinum ineligibility. One would argue that [the parameters are] tight and certainly what we were doing internally. So a creatinine clearance less than 30 mL/min and an ECOG function more than or equal to 3 [is the definition of platinum eligibility from this survey].
[Another way to define bladder cancer is through] the Galsky criteria by Matt Galsky, MD, of Mount Sinai School of Medicine. He surveyed a much laxer group [of oncologists]. The criteria were a performance status of 2 and a creatinine clearance of 60 mL/min. When thinking of patients with hypertensive diabetes, how many of them are in the 56 to 60 mL/min [range]? He also weaved in hearing loss, peripheral neuropathy, and heart failure. So there are 2 competing ways of looking at platinum eligibility.
Now, [there are] great drugs [compared with what used to be available beyond platinum therapy]. We see that as a group, and people are more [often] following along with Galsky’s criteria. [Some are] asking for even better than Galsky’s criteria. For most clinical trials, the definition of platinum ineligibility at the current time is going by the Galsky criteria. The easiest thing for me to remember is a [definition of a] creatinine clearance of 60 mL/min.
ECOG performance status can change quickly, sometimes in the eye of the beholder. So I certainly use creatinine clearance more often [as criterion for platinum eligibility] because ECOG performance status is sometimes a little shaky to me.

What do you think of this patient’s updated case?
This patient seemed to be platinum and cisplatin eligible. She had 4 cycles of cisplatin. We can go back and forth [on whether to use 4 or 6 cycles], but nevertheless, some version of platinum was used.
She discontinued because of toxicity. In my experience, I think the neuropathy really gets to patients, and the fatigue is sometimes difficult [for them.] When she was rescanned, there was no deep response but an SD, so probably nodes [in the bladder] and lung [metastases] look the same.
This patient was a woman who got SD as their deepest response when given her best option, which was platinum chemotherapy.
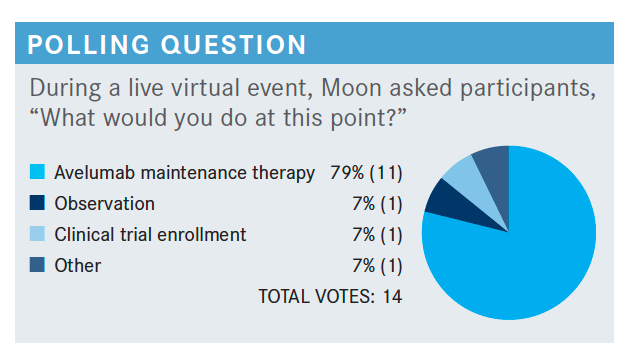
Do you agree with these poll results?
I think there’s soon to be a plurality of patients going on to avelumab maintenance therapy. [Choosing] observation I can see, especially if a patient is pretty beat up and tired in the whole situation; sometimes the patient drives that conversation. Clinical trials [are a reasonable choice], if this is a built-in behavior that you do [to send a patient to a clinical trial], I think that makes sense.
What are the trial data for avelumab maintenance in patients with bladder cancer?
There are a number of JAVELIN trials, so the JAVELIN Bladder 100 cues the fact that it was a switch maintenance trial.1 The agent here was avelumab, which is a checkpoint inhibitor, after first-line platinum therapy. It could have been carboplatin or cisplatin for patients with advanced urothelial carcinoma. Patients were allowed up to—because there are community standards—4 to 6 cycles. If they did not progress, they were able to go on to this maintenance therapy within 4 to 10 weeks. Sometimes, if a patient wanted a little time off, [it was] 10 weeks; sometimes, that may be a relevant point.
There were 700 patients [in the trial] and what should be noted is that this treatment was until progression, so there was no set end date; it wasn’t a year and done. [Treatment didn’t end] until progression, unacceptable toxicity, or withdrawal. It’s one of the studies whose primary end point was OS [overall survival], and they subsequently separated groups by different disease [factors], how many cycles, checkpoint status, and so on and so forth.
What was the efficacy of JAVELIN Bladder 100?
This was the first trial in bladder cancer to show OS.2 The median OS for avelumab plus best supportive care [BSC] was 21.4 months [HR, 0.69; 95% CI, 0.56-0.86; P <.001]. For those of us who look at bladder data a lot, this is impressive as most of the bladder survival data are in the [13 to 19-month range]. When looking at the second line, pembrolizumab [Keytruda] combination data where they’re trying to bring the chemotherapy plus immune-oncology upfront, no one has ever gone beyond 20 months, basically.
When looking at BSC alone, presumably they got platinum therapy, and then, what we usually do, is let them recover [and observe]. That’s our standard of care. [Investigators are] seeing that number reflected at 14.3 months, and it’s a hazard ratio of 0.69 that’s highly significant.
There was an objective response rate of 9.4 versus 1.4 months. The investigators [separated the group of patients who had] PD-L1 positive status. In order to get into this trial, the patient had to already [have responded.] What they were really trying to say is if a response deepened—so if somebody was in complete response [CR] when they wandered into this trial—that’s it, they could not get a deeper CR. Did a partial response [PR] then turn into a CR? Did someone whose standard was SD turn into [PR or CR]?
In general, what the investigators were saying was that every subgroup favored avelumab plus BSC. There were a couple of groups that crossed that midline of 1.00. The best response [to first-line chemotherapy] of SD or CR/PR attempts to pull out the most important [aspect of] creatinine clearance. In some ways, if using [creatine clearance] as a surrogate [end point] for using cisplatin versus carboplatin, [the efficacy was similar]. The PD-L1 positive population did better, but patients could still respond if they had negative expression. That’s the general idea. In this particular cohort of patients, it didn’t make as much [of a difference].
There was an additional subgroup analysis. For bladder cancer, one of the things that’s relevant is hepatic metastasis. Generally, if the patient has liver metastases, they go downhill quickly. This analysis tried to show at least some idea that [nothing veered from favoring avelumab], even though one would argue it crosses the midline in some patients. But metastasis does have a negative impact on survival in general.
Can you describe the toxicity profile with avelumab?
[As far as] treatment-emergent adverse events [AEs]…I don’t think they were surprising for any of us. One of the things I’m looking at for this trial is discontinuation rates. [Some] patients and their families want to keep going. The 11.9% that discontinued does not seem unusually high to me.
There was a small death rate; 2 patients with sepsis and ischemic stroke, respectively. One of the things we talk about is that patients with bladder cancer tend to be smokers; it’s a smoking cancer, and sometimes the ischemia [can cause] heart attacks. It’s an issue, so whether they’re related or not, the physician can decide on that AE signal. There were no grade 4 or 5 immune-related AEs, and steroids were used to salvage about 9% of patients. In general, I find avelumab is not a very challenging medication to give.
Has avelumab been integrated into practice at this point?
Based on these data, last summer, the FDA approved avelumab for urothelial cancer maintenance therapy, more colloquially called switch maintenance therapy.3 The idea is that you would not wait for progression, which is called second-line therapy, but the patient would continue with the maintenance therapy.
This similarly led to the National Comprehensive Cancer Network [adding it into the guidelines] around [the time of] approval.4 A 2021 American Society of Clinical Oncology Genitourinary Symposium abstract retrospectively looked at the duration of first-line chemotherapy.5 Some of the genitourinary oncologists really [placed importance] on 4 versus 6 cycles and functional status. [Whether a physician uses] 4, 5, or 6 cycles, the patients do no worse, even though for some reason, at 5 cycles, the Kaplan- Meier curves were close but maybe [only] because there were very few patients. But there’s a benefit with 4 or 6 cycles.
References:
1. Powles T, Park SH, Voog E, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. J Clin Oncol. 2020;38(18 suppl):LBA1. doi:10.1200/JCO.2020.38.18_ suppl.LBA1
2. Powles T, Park SH, Voog E, at al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. doi:10.1056/NEJMoa2002788=
3. FDA approves avelumab for urothelial carcinoma maintenance treatment. News release. FDA. June 30, 2020. Accessed April 7, 2021. https://bit.ly/3jaforD
4. NCCN. Clinical Practice Guidelines in Oncology. Bladder cancer, version 2.2021. Accessed April 7, 2021. https://bit.ly/3rQRjtr
5. Loriot Y, Powles T, Durán MAC, et al. Avelumab (Ave) first-line (1L) maintenance plus best supportive care (BSC) versus BSC alone for advanced urothelial carcinoma (UC): JAVELIN Bladder 100 subgroup analysis based on duration and cycles of 1L chemotherapy. J Clin Oncol. 2021;39(suppl 6):438. doi:10.1200/ JCO.2021.39.6_suppl.438

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More