Previous Liver Infection Requires a Rapid and Flexible Treatment Response in NSCLC
Jorge A. Rios, MD, a medical oncologist at Zangmeister Cancer Center in Columbus, Ohio, part of the American Oncology Network, shared the case of a woman who developed multiple severe adverse events attributable to durvalumab.
Jorge A. Rios, MD, a medical oncologist at Zangmeister Cancer Center in Columbus, Ohio, part of the American Oncology Network, shared the case of a woman who developed multiple severe adverse events (AEs) attributable to durvalumab (Imfinzi). The 57-year-old woman has a history of obesity, anxiety, and latent tuberculosis (TB) and is currently on therapy receiving isoniazid. She had no history of transfusions, tattoos, use of intravenous drugs, or high-risk behaviors for sexually transmitted diseases.
As part of the work-up for the latent TB, she had a CT scan of the chest on April 2019 that indicated a lung nodule in the right upper lobe (RUL). Follow-up on December 2019 showed an enlarging RUL nodule, which measured 1.5 cm at the time (spiculated), as well as a second nodule on the right lower lobe (RLL). PET/CT imaging showed increased 18F-fluorodeoxyglucose (FDG) uptake on both RUL (maximum standardized uptake value [SUVmax], 11) and RLL (SUVmax, 3.6) nodules, as well as FDG uptake in the mediastinum. There was no evidence of FDG uptake outside the chest. A biopsy of the RUL nodule showed adenocarcinoma of lung origin.
A multidisciplinary team deemed the patient ineligible for surgical resection. She started concurrent chemoradiation with carboplatin and paclitaxel, and transitioned to durvalumab on April 22, 2020.
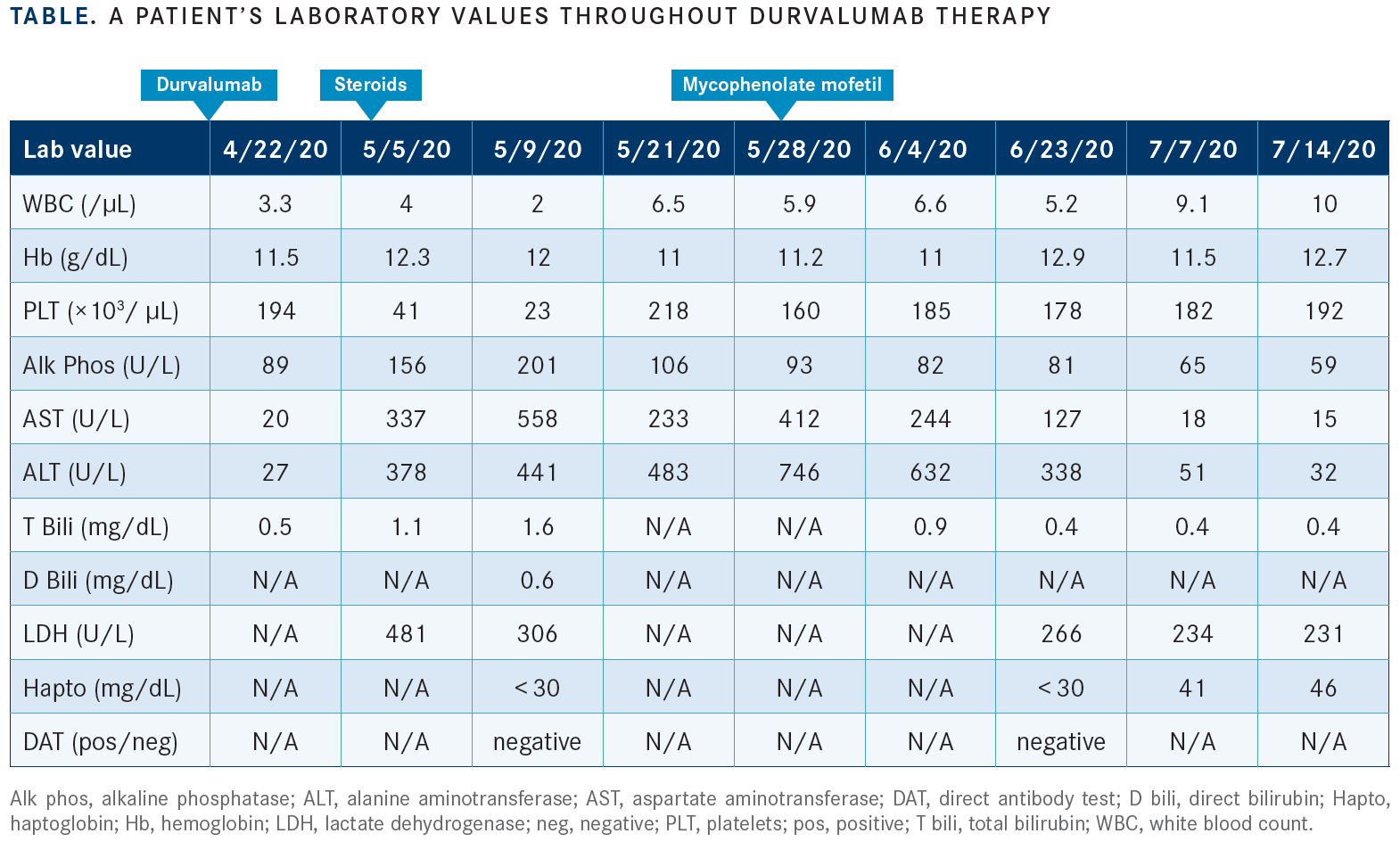
Baseline laboratory work-up showed no abnormalities on the complete blood count or complete metabolic panel. Seven days after receiving durvalumab, the patient developed multiple symptoms including diffuse musculoskeletal symptoms, nausea, and ecchymosis. In an unscheduled visit to the office, her platelets dropped from 194 × 103/μL prior to durvalumab to 41 × 103/μL. Liver chemistries showed an increase in her aspartate aminotransferase levels from 28 U/L to 337 U/L, and alanine aminotransferase levels from 27 U/L to 378 U/L. Creatine phosphokinase was normal. The hemoglobin level decreased from 12.4 g/dL to 11.0 g/dL. Her serum lactate dehydrogenase was increased at 481 U/L; haptoglobin became undetectable as determined by a negative direct antiglobulin test.
Durvalumab and isoniazid were held. An ultrasound of the abdomen showed no abnormalities on the patency of the hepatic vessels, with flow in the appropriate directions. She had evidence of prior infection for hepatitis B (positive hepatitis B core antibody IgM, positive hepatitis B antibody, negative hepatitis B surface antigen, positive hepatitis B surface antibody. Hepatitis B viral DNA was nondetectable by PCR). Her ferritin level was at 302 ng/mL.
There was no evidence of antimitochondrial antibodies or anti–smooth muscle antibodies on the screening test. The antinuclear antibody screen was also negative. The patient declined a liver biopsy.
She was started on high-dose oral steroids at 1 mg/kg/ day of prednisone with close monitoring. After an initial modest improvement in the liver chemistries with high-dose steroids, she experienced a worsening of the liver chemistries. As a result, mycophenolate mofetil (CellCept) was added. With this intervention, her liver chemistries improved gradually. Over the span of 10 weeks, the liver chemistries and platelets normalized. The TABLE illustrates some of the patient’s laboratory values to demonstrate how her case evolved.
Durvalumab
Based on results from the phase 3 PACIFIC trial (NCT02125461), the FDA approved durvalumab for the treatment of patients with locally advanced, unresectable, stage III non–small cell lung cancer (NSCLC) who have not progressed following chemoradiotherapy.
The PD-L1 inhibitor improved median progression-free survival (PFS) by 11.2 months compared with placebo (16.8 vs 5.6 months; HR, 0.52; 95% CI, 0.42-0.65; P < .0001).1 The 12-month PFS rate was 55.9% versus 35.3%, and the 18-month PFS rate was 44.2% versus 27.0%, again favoring the durvalumab arm.
PACIFIC took place at 235 centers in 26 countries, with 473 patients randomized to durvalumab and 236 randomized to placebo. PFS was the primary end point, along with overall survival (OS).
The median age of patients in the study was 64 years, and most were current or former smokers (91%). The majority were men (70.1%), and most had squamous histology (45.7%). Chemotherapy use was similar between the groups, with 25.8% and 28.7% receiving induction therapy before definitive chemoradiotherapy, in the durvalumab and placebo groups, respectively. Corresponding responses to chemoradiotherapy was similar between the 2 arms, with objective response rates (ORRs) of 50.6% and 49.8%.
The PFS benefit associated with durvalumab was consistent across all prespecified subgroups, as defined according to patient demographic characteristics, baseline clinicopathologic features, and response to previous treatment. The PFS benefit held irrespective of PD-L1 expression before chemoradiotherapy. The hazard ratio was 0.59 (95% CI, 0.43-0.82) for patients with a PD-L1 expression level less than 25%, and the HR was 0.41 (95% CI, 0.26-0.65) for patients with a PD-L1 expression level of 25% or greater.
ORR, as assessed by blinded independent central review, was also significantly higher with durvalumab (28.4% vs 16.0%; P < .001). Of the patients who had a response to durvalumab, 72.8% had an ongoing response at both 12 and 18 months compared with 56.1% and 46.8%, respectively, in the placebo arm.
Just 16.5% of patients in the durvalumab group experienced disease progression compared with 27.7% of the placebo group (P < .001).
Nearly all patients in both groups, 96.8% for durvalumab and 94.9% for placebo, experienced AEs of any cause and grade. Grade 3/4 AEs were slightly more common with durvalumab (29.9% vs 26.1%). Pneumonia was the most common grade 3/4 AE, and was observed in 4.4% of patients in the durvalumab group and 3.8% of patients in the placebo group.
AEs caused treatment discontinuation in 15.4% of patients in the durvalumab group and in 9.8% of the placebo group. About 29% of patients in the durvalumab group experienced serious AEs compared with 22.6% of the placebo arm.
Most frequent AEs leading to discontinuation were pneumonitis or radiation pneumonitis and pneumonia in both groups. One-third of patients assigned to durvalumab experienced any-grade pneumonitis or radiation pneumonitis compared with 24.8% in the placebo group. Grade 3/4 pneumonitis or radiation pneumonitis occurred in 3.4% of patients receiving durvalumab and 2.6% of the placebo group. Deaths due to AEs occurred in 4.4% of patients on durvalumab and 5.6% in the placebo group.
Multiple Subspecialties
This case emphasizes the multidisciplinary nature of cancer care. It encompassed the work of multiple subspecialties (oncology, gastroenterology, infectious diseases) and disciplines (nursing, pharmacy) working together to treat the patient.
The case highlights the importance of close monitoring during treatment, as well as the value of having prompt access to outpatient services and rapid transitioning to inpatient care when needed. Most of the patient’s care was delivered in the outpatient setting with close communication between clinicians and rapid change in the treatment plan according to need.
Although immune-mediated AEs related to the use of immune checkpoint inhibitors is a well described occurrence, it is unusual for the patient to present with more than 1 suspected immune-mediated event at the same time.
Furthermore, most of the clinical trials that led to the approval of these drugs in cancer care excluded patients with autoimmune disorders or certain infections, but a growing body of literature shows the feasibility of the use of these medications in those populations. This has opened up the possibility of extending the use of these medications in the “real world” setting.
For this particular patient, the infectious diseases and hepatology specialists’ role was paramount given that there were several potential causes for her liver chemistry abnormalities, some of which were uncovered when she had the liver injury.
Joint decision making on how to handle latent TB, unknown past hepatitis B infection, worsening liver function despite steroid use, and worsening cytopenias in the midst of immune checkpoint inhibitor use was possible because of the close communication and coordination of care between specialists in infectious diseases, hepatology, and medical oncology, along with supportive staff including nursing and pharmacy.
Reference
Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non—small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi:10.1056/NEJMoa1709937