PARP Inhibitors Gain Indications in Advanced Prostate Cancer
PARP inhibitors have propelled the field of metastatic castration-resistant prostate cancer forward as the next class of therapeutics to advance outcomes.
Emmanuel Stylianos Antonarakis, MBBCh

In recent months, PARP inhibitors have propelled the field of metastatic castration-resistant prostate cancer (mCRPC) forward as the next class of therapeutics to advance outcomes. Approvals for 2 PARP agents, along with data from major medical conferences regarding the use of immunotherapeutics and other anticancer agents, show promise for the treatment of patients with advanced disease.
“We always knew that it was a matter of time for PARP inhibitors to get approved for patients with metastatic prostate cancer,” said Neeraj Agarwal, MD, in an interview with Targeted Therapies in Oncology (TTO). He is a professor in the Division of Oncology, Department of Medicine, at the University of Utah School of Medicine, and director of the Genitourinary Oncology Program at the Huntsman Cancer Institute in Salt Lake City.
Neeraj Agarwal, MD
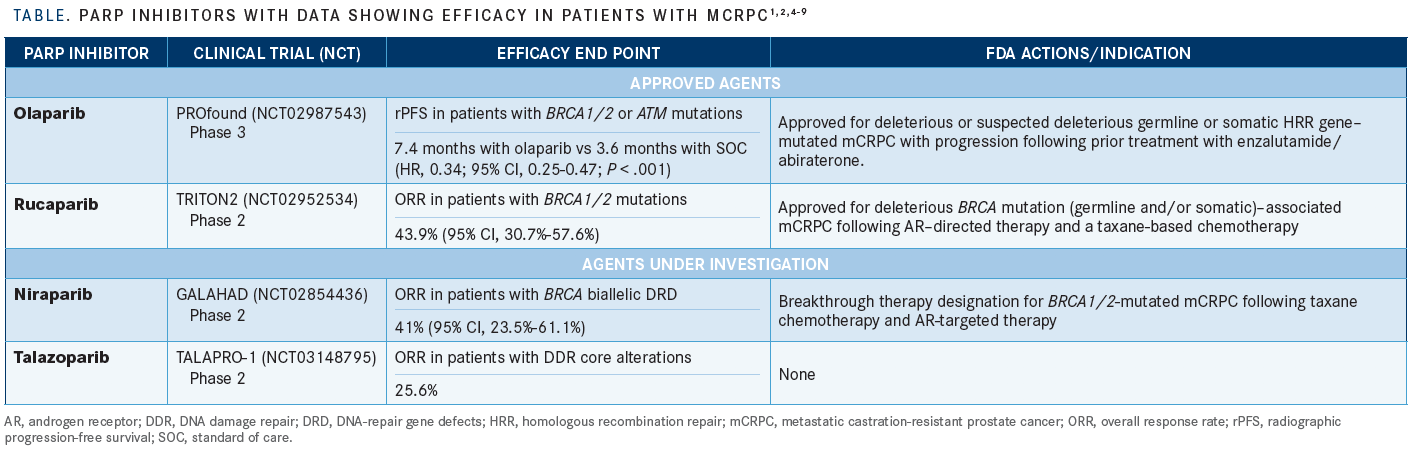
The PARP inhibitor olaparib (Lynparza) gained FDA approval for treatment of mCRPC in May 2020, and was the first approval for this class of agent in prostate cancer with supporting efficacy data based on a randomized phase 3 clinical trial. The PROfound trial (NCT02987543) demonstrated improved radiographic progression-free survival (rPFS) with olaparib versus either enzalutamide (Xtandi) or abiraterone acetate (Zytiga) in patients whose mCRPC had deleterious or suspected deleterious germline or somatic mutations in homologous recombination repair (HRR) genes (7.4 months vs 3.6 months; HR, 0.34; P <.001).1,2
“These were remarkable findings...olaparib was clearly superior by almost 4 months,” Agarwal said.
PARP Inhibitors in Advanced Prostate Cancer
PARP enzymes play a critical role in repairing single-strand DNA breaks. With the use of PARP inhibitors, these lesions remain unrepaired and often progress to double-strand breaks (DSBs), which are preferentially resolved by HRR. However, germline or somatic homologous recombination deficiency (HRD), which is present in up to 25% of patients with mCRPC, obliges tumor cells to repair DSBs via an error-prone alternative pathway, nonhomologous end joining (NHEJ). The genetic aberrations introduced by NHEJ often irreparably damage key cellular pathways, dooming cancer cells to apoptosis—hence the keen interest in treating tumors with HRD using PARP inhibitors.3 With limited treatment options for mCRPC, the development of PARP inhibitors has provided an important lifeline in this population, especially in the HRD setting (TABLE).1,2,4-9
Olaparib
The pivotal PROfound study was a prospective, randomized, multicenter, open-label, phase 3 clinical trial that randomized patients 2:1 to receive either olaparib or androgen receptor–targeted therapy (ART) consisting of enzalutamide or abiraterone. Patients with mutations in HRR genes BRCA1/2 or ATM made up cohort A (olaparib group, n = 162; ART group, n = 83). Patients with a mutation in any of 12 other HRR genes were treated in cohort B (olaparib; n = 94; ART, n = 48).2
“The secondary analysis looking at additional genes [in cohort B] was exploratory; however, the exploratory analysis was only possible and statistically permitted if the primary analysis for BRCA1/2 and ATM was positive, which it was,” Emmanuel Stylianos Antonarakis, MBBCh, professor of oncology and urology at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, Maryland, said in an interview with TTO. After the primary end point of rPFS in cohort A was met, a review of both cohorts combined demonstrated improvement in rPFS, at 5.8 months for olaparib versus 3.5 months with ART (HR, 0.49; 95% CI, 0.38-0.63; P < .001).2
Additionally, an interim analysis showed that median overall survival (OS) in cohort A was 18.5 months with olaparib versus 15.1 months with ART (HR, 0.64; 95% CI, 0.43-0.97; P = .02). This difference did not achieve statistical significance, however, due to the preset cutoff of P ≤ .01 for this data point.
“Patients who were randomized to the enzalutamide or abiraterone arm were allowed to cross over to the olaparib arm at the time of disease progression, and a large number of patients [82%] actually crossed over. Despite the crossover, the median OS [benefit] remained significant, at 3 months higher compared with abiraterone or enzalutamide,” Agarwal said. The interim median OS analysis of both cohorts significantly favored olaparib, at 17.5 months versus 14.3 months with ART (HR, 0.67; 95% CI, 0.49-0.93; P = .0063).2
Other secondary end points, including objective response rate (ORR) and median time to pain progression in cohort A, also significantly favored olaparib. Common adverse events (AEs) that occurred with olaparib included anemia, nausea, and fatigue or asthenia. Additionally, grade 3 or higher AEs occurred more frequently with olaparib compared with ART.2
“I think the PROfound trial is the first large phase 3 randomized trial that showed a PARP inhibitor is superior to these standard-of-care [therapies] for patients who have disease progression on abiraterone or enzalutamide and are harboring mutations in DNA repair genes. It’s a very exciting development that’s happened in the last few months,” Agarwal said.
Further support for olaparib use in mCRPC was demonstrated in the phase 2 TAPUR trial (NCT02693535). Patients in this trial had exhausted all standard options, harbored germline or somatic BRCA1/2 mutations, had an ECOG performance score of 2 or less, had measurable disease, and had adequate organ function. The disease control rate was 68% (90% CI, 53%-77%) in the 25 patients evaluated. The interim median OS was 75.3 weeks (95% CI, 49.1-88.9). The results of this study demonstrated the utility of olaparib as a therapy option in patients with heavily pretreated mCRPC harboring germline or somatic BRCA1/2 mutations.10
Rucaparib
Another PARP inhibitor that recently received accelerated approval for mCRPC is rucaparib (Rubraca).4 The TRITON2 trial (NCT02952534) showed encouraging antitumor activity of rucaparib in 57 patients with BRCA1/2 mutations, with an ORR of 43.9% (95% CI, 30.7%-57.6%).5
There were 2 distinct differences between the patient groups approved for this PARP inhibitor and for olaparib, Agarwal indicated. The first was treatment history; consistent with the PROfound trial’s eligibility requirements, olaparib approval specified that patients need only have progressed on ART. “Rucaparib was approved based on the TRITON2 trial, in which patients had received both abiraterone or enzalutamide and docetaxel chemotherapy,” Agarwal noted.
“The number of eligible genes also differs. Rucaparib is only approved for BRCA1/2 [mutations],” Agarwal added, which contrasts with olaparib’s approval for mCRPC associated with mutations in multiple HRR genes.
Antonarakis also noted some differences in the AE profiles of the 2 drugs: “The adverse effects that grabbed my attention [that] were present with olaparib and perhaps not with rucaparib were…a slightly higher risk of venous thromboembolic events…and a rare, but serious, [risk of] pneumonitis.”
Additional PARP Inhibitors on the Horizon
Other PARP inhibitors that show potential for FDA approval in mCRPC include niraparib (Zejula) and talazoparib (Talzenna). Niraparib is being explored in the setting of BRCA1/2 mutations after disease progression on ART and taxane chemotherapy. It was granted breakthrough therapy designation by the FDA based on the phase 2, multicenter, open-label GALAHAD trial (NCT02854436).6 Based on data presented at the European Society for Medical Oncology 2019 Congress, the agent demonstrated a 41% ORR in patients with mCRPC who have BRCA biallelic DNA-repair gene defects.7
Talazoparib has also demonstrated benefit in patients with mCRPC driven by alterations in BRCA1/2 or other DNA damage repair genes after treatment with at least 1 taxane chemotherapy regimen and ART. An interim analysis of the TALAPRO-1 trial (NCT03148795) showed an ORR of 25.6% (95% CI, 13.5%-41.2%) in the overall cohort. The ORR was higher, at 50.0% (95% CI, 27.2%-72.8%), in the subset of patients with BRCA1/2 mutations.8
Commenting on the future of PARP inhibitors, Agarwal predicted that “there will be a time soon [when] we will be testing these PARP inhibitors in the up-front setting in patients with newly diagnosed prostate cancer who are harboring mutations in DNA repair genes.”
With the recent approvals of PARP inhibitors, the National Comprehensive Cancer Network has updated its guidelines to recommend tumor testing for HRR mutations. Additionally, both olaparib and rucaparib have been added to the list of systemic therapy options for patients with HRR-mutated mCRPC to reflect the current standard of care.10
Other Agents Move Forward
Antonarakis noted that in addition to PARP inhibition, “one of the most exciting developments [in prostate cancer treatment is the use of] radioligand treatment that targets prostate-specific membrane antigen [PSMA]–expressing prostate cancer. PSMA is almost ubiquitous in prostate cancer in castration-resistant disease and is present in more than 80% to 90% of castration-resistant metastasis. It’s a very reasonable target.”
The phase 2 TheraP trial (NCT03392428) randomized patients with mCRPC who had progressed after docetaxel to treatment with either lutetium 177–labeled PSMA-617 (177Lu-PSMA-617; LuPSMA) or cabazitaxel (Jevtana; a taxane). Antonarakis explained that Lu-PSMA “is a radioligand whereby a radioactive molecule, lutetium, gives off a type of radiation called b waves [and] binds to prostate cancer cells that are expressing PSMA. The lutetium is used to irradiate the cells that it binds.”
Patients were randomized to either Lu-PSMA (n = 98) every 6 weeks for up to 6 cycles or cabazitaxel (n = 85) every 3 weeks for up to 10 cycles. The percentage of patients meeting the primary end point of prostate-specific antigen (PSA) response rate (PSA reduction ≥50% from baseline) was almost doubled in the Lu-PSMA group compared with the cabazitaxel arm, at 66% versus 37%, respectively (P < .0001). A sensitivity analysis also favored the experimental arm and demonstrated a 23% difference in PSA response rate (P = .0016). Preliminary results demonstrated improved PSA PFS (defined as time from randomization to PSA progression) at a median follow-up of 13.3 months (HR, 0.69; 95% CI, 0.50-0.95; P = .02).11
Additionally, grade 3/4 AEs occurred less frequently in the Lu-PSMA group (35%) compared with the cabazitaxel group (54%). Diarrhea, dysgeusia, and neuropathy of any grade occurred more frequently with cabazitaxel, whereas thrombocytopenia, dry mouth, and dry eye occurred more frequently with Lu-PSMA. Results of this study indicate that Lu-PSMA represents a potential novel class of effective therapy for mCRPC after progression on taxane chemotherapy.11
“The theoretical advantage of Lu-PSMA…is that it will target bone and nonbone lesions—for example, soft tissue metastases,” Antonarakis said. “The phase 3 study with Lu-PSMA [VISION trial; NCT03511664] is awaiting results. Many of us in the field anticipate that Lu-PSMA will be FDA approved by the middle of 2021.”
Another notable study is the CARD trial (NCT02485691), which evaluated the use of cabazitaxel (n = 129) versus ART (n = 126; abiraterone or enzalutamide) after previous docetaxel and within 12 months of progression on ART. This was a multicenter, randomized, open-label trial with rPFS as the primary end point. The median follow-up was 9.2 months. Cabazitaxel improved median rPFS by more than 4 months compared with ART, at 8.0 months versus 3.7 months (HR, 0.54; 95% CI, 0.40-0.73; P < .0001). Additionally, cabazitaxel improved median OS compared with ART, at 13.6 months versus 11.0 months (HR, 0.64; 95% CI, 0.46-0.89; P = .0078).12
Furthermore, analyses evaluating OS from various starting points—including diagnosis of metastatic disease, diagnosis of mCRPC, and initiation of first and second life-extending therapies—all favored cabazitaxel over ART. The survival benefit of cabazitaxel compared with ART was supported by a stratified multivariate analysis. Results of the CARD study support cabazitaxel over ART as the standard of care for patients previously treated with docetaxel and ART.12
“What the CARD trial has taught us is that we should alternate ART with taxane chemotherapy,” Antonarakis said.
The pivotal CARD trial inspired the creation of other studies to evaluate different end points and patient populations. One of these studies was PROREPAIR-B (NCT03075735), a prospective trial evaluating the effects of germline mutations in HRR genes on the benefit of first, second, and subsequent lines of therapy. Of the 419 patients in this trial, 95 met the same inclusion criteria as those for the CARD study.
In the overall population, cabazitaxel (n = 60) demonstrated improved rPFS compared with ART (n = 35; abiraterone/enzalutamide), at 6.0 months versus 3.7 months, respectively (P = .03). No difference in OS was detected, perhaps due in part to the 60% rate of crossover from ART to cabazitaxel. However, subgroup analyses revealed that patients with germline HRR mutations had a significantly worse prognosis, with poor hazard ratios for OS (HR, 1.9) and rPFS (HR, 2.4), as well as no improvement in OS (4.5 months vs 3.7 months; P = .8) or rPFS (2.5 months vs 3.8 months; P = .8) with cabazitaxel compared with ART. The PROREPAIR-B findings further support the results of the CARD trial for unselected patients, favoring the use of cabazitaxel over ART.13
However, the poor outcomes with both regimens in patients with germline HRR–mutant mCRPC support the need for novel treatments in this setting.13 “There is accumulating evidence, although it’s not conclusive yet, that perhaps patients with HRR mutations—especially BRCA2 and particularly when [it is a BRCA2] germline mutation—may actually be a bit more taxane resistant and more hormone therapy sensitive, which might sound like a bit of a paradox,” Antonarakis said. “The [data] are immature but provocative…and should be further studied.”
In a preplanned analysis of the CARD trial, patient-reported outcomes were evaluated using a questionnaire, the EuroQol Group’s 5-dimension, 5-level (EQ-5D-5L) utility index score and visual analogue scale (VAS). The investigators evaluated the scores at baseline, on day 1 of each cycle, and at the end of treatment. At baseline, patients randomized to the cabazitaxel versus ART groups had similar EQ-5D-5L utility index scores (0.7 in both groups) and VAS (65.8 with cabazitaxel vs 66.3 for ART). Pain response (a reduction in pain from baseline) was reported in a significantly greater proportion of the cabazitaxel group (45.9%) compared with the ART group (19.3%; P < .0001). Cabazitaxel also delayed the time to pain progression compared with ART (HR, 0.55; 95% CI, 0.32-0.97; P = .03). Additionally, the safety profile of cabazitaxel was manageable, with fewer grade 3 or higher cardiac disorders compared with ART (0.8% vs 4.8%).14
As another novel approach for treating mCRPC, immunotherapeutics are making their way into the treatment landscape. Agarwal remarked that because “cabozantinib [Cabometyx] and atezolizumab [Tecentriq] have synergistic action…they were found to suppress the immune-suppressive cells and upregulate immunostimulatory cells.”
This was the rationale for exploring the small-molecule multityrosine kinase inhibitor/PD-L1 inhibitor combination of cabozantinib and atezolizumab in the phase 1b COSMIC-021 (NCT03170960) trial, with multiple cohorts of patients with 1 of 12 types of advanced solid tumors, including mCRPC. Patients enrolled in cohort 6 had mCRPC with radiographic progression in soft tissue after ART, measurable disease, and an ECOG performance status of 0 or 1. The primary end point of ORR was 32% (80% CI, 23%-42%) for the first 44 patients enrolled, including 3 patients with complete responses and 11 who achieved partial responses. Furthermore, 21 patients (48%) achieved stable disease, for a disease control rate of 80%.15
Commenting on the study results, Agarwal stated that “we have never seen this magnitude of response in the context of any immunotherapy in prostate cancer in the metastatic setting in the history of castration-resistant prostate cancer.” Additionally, the median time to objective response was 1.6 months (range, 1-7), and the median duration of response was 8.3 months (range, 2.8-12.5+). Agarwal noted, “These responses were not only very high in number…but they were also durable. That is the other exciting aspect of this regimen: Once patients responded, they were likely to continue to respond.”
The majority (82%) of patients enrolled had high-risk mCRPC (with visceral and/or extrapelvic lymph node metastases), and the median age was 70 years (range, 49-90). The most common treatment-related AEs were fatigue, diarrhea, nausea, decreased appetite, and dysgeusia.15
Looking Ahead
The landscape of mCRPC is rapidly evolving. These new treatment modalities—including PARP inhibition, PSMA-targeting agents, and immunotherapy—are shifting the treatment paradigm in mCRPC and providing patients with more therapeutic options.
“The field is...evolving in 2 key directions in mCRPC. One will be targeted therapy, and [the other] will be immunotherapy,” said Antonarakis. “I think overall, the future looks promising, and I tell my patients I need to keep them going only for the next few years because after that, we will see a new wave of therapy coming up.”
Antonarakis discussed the future of mCRPC in terms of novel approaches to treatment. “Perhaps [with] combination strategies using PD-1 inhibitors or other modalities, such as PSMA-targeted BiTEs, or bispecific T-cell engagers, or PSMA CAR [chimeric antigen receptor] T cells,” he said, “we might have a drug that will [further] improve outcomes for our patients.”
References
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA. Updated May 20, 2020. Accessed August 5, 2020. https://bit.ly/3ic71dD
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
- Christenson ES, Antonarakis ES. PARP inhibitors for homologous recombination-deficient prostate cancer. Expert Opin Emerg Drugs. 2018;23(2):123-133. doi:10.1080/14728214.2018.1459563
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. FDA. May 15, 2020. Accessed July 27, 2020. https://bit.ly/3kjRf2m
- Abida W, Campbell D, Patnaik A, et al. 846 PD: preliminary results from the TRITON2 study of rucaparib in patients (pts) with DNA damage repair (DDR)-deficient metastatic castration-resistant prostate cancer (mCRPC): updated analyses. Ann Oncol. 2019;30(suppl 5):v327-v328. doi:10.1093/annonc/mdz248.003
- Janssen announces U.S. FDA breakthrough therapy designation granted for niraparib for the treatment of metastatic castration-resistant prostate cancer. News release. Johnson & Johnson; October 3, 2019. Accessed July 27, 2020. https://bit.ly/2XtVheI
- Smith MR, Sandhu SK, Kelly WK, et al. Pre-specified interim analysis of GALAHAD: a phase II study of niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD). Ann Oncol. 2019;30(suppl 15):v884-v885. doi:10.1093/annonc/mdz394
- De Bono JS, Mehra N, Higano CS, et al. TALAPRO-1: a phase II study of talazoparib (TALA) in men with DNA damage repair mutations (DDRmut) and metastatic castration-resistant prostate cancer (mCRPC)—first interim analysis (IA). J Clin Oncol. 2020;38(suppl 6; abstr 119). doi:10.1200/JCO.2020.38.6_suppl.119
- Pisick E, Garrett-Mayer E, Halabi S, et al. Olaparib (O) in patients (pts) with prostate cancer with BRCA1/2 inactivating mutations: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J Clin Oncol. 2020;38(suppl 15; abstr 5567). doi:10.1200/JCO.2020.38.15_suppl.5567
- NCCN. Clinical Practical Guidelines in Oncology. Prostate cancer, version 2.2020. Accessed August 1, 2020. https://bit.ly/2Dr8jT8
- Hofman MS, Emmett L, Sandhu SK, et al. TheraP: a randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: initial results (ANZUP protocol 1603). J Clin Oncol. 2020;38(suppl 15; abstr 5500). doi:10.1200/JCO.2020.38.15_suppl.5500
- Tombal BF, Castellano D, Kramer G, et al. CARD: overall survival (OS) analysis of patients with metastatic castration-resistant prostate cancer (mCRPC) receiving cabazitaxel versus abiraterone or enzalutamide. J Clin Oncol. 2020;38(suppl 15; abstr 5569). doi:10.1200/JCO.2020.38.15_suppl.5569
- Romero-Laorden N, Lozano R, Llacer Perez C, et al. Cabazitaxel versus enzalutamide/abiraterone in CARD eligible mCRPC patients with or without germline HRR defects. J Clin Oncol. 2020;38(suppl 15; abstr 5554). doi:10.1200/JCO.2020.38.15_suppl.5554
- Kramer G, Sternberg CN, Fizazi K, et al. PD16-08: effect of cabazitaxel vs abiraterone or enzalutamide on patient-reported outcomes in metastatic castration-resistant prostate cancer: a pre-planned EQ-5D-5L analysis of the CARD study. J Urol. 2020;203(suppl 4):e366. doi:10.1097/JU.0000000000000859.08
- Agarwal N, Loriot Y, McGregor BA, et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results of cohort 6 of the COSMIC-021 study. J Clin Oncol. 2020;38(suppl 15; abstr 5564). doi:10.1200/JCO.2020.38.15_suppl.5564