Deferred Cytoreductive Nephrectomy Plus Sunitinib Bests Immediate Surgery in RCC
In a post hoc analysis of the randomized phase 3 SURTIME trial of patients with metastatic renal cell carcinoma, early treatment with sunitinib demonstrated better control over the disease as well as detection of progression before planned cytoreductive nephrectomy.

In a post hoc analysis of the randomized phase 3 SURTIME trial (NCT01099423) of patients with metastatic renal cell carcinoma (RCC), early treatment with sunitinib (Suntent) demonstrated better control over the disease as well as detection of progression before planned cytoreductive nephrectomy (CN), which could translate to the survival benefit seen in the original study, according to a presentation from Yasmin Abu-Ghanem, MD, MSc, at the 35th Annual European Association of Urology Virtual Congress.1
Investigators also found that fewer patients received systemic therapy with sunitinib after immediate CN and had therapy administered later and for shorter periods of time compared with patients who received sunitinib, then the deferred CN, followed by continued sunitinib afterward.
For this study, the investigators looked at the differences in response when patients were given upfront systemic therapy and then deferred CN compared with immediate CN, and which of these responses was deeper at metastatic sites. If it was deeper for patients in the deferred arm, it would explain the observed overall survival (OS) benefit for patients who received deferred surgery.2
The results showed that 19.6% of patients in the immediate arm had confirmed progression 4 weeks after surgery compared with baseline. Twenty-five percent of patients started sunitinib over a month after immediate CN.1
At 16 weeks, 46.0% of patients who underwent immediate CN had progressed at metastatic sites compared with 32.7% who deferred surgery. Eighty percent and 97.7% of patients received sunitinib in each arm, respectively.
“When we compared the length of drug exposure, we found that more patients received sunitinib in the deferred arm for a longer period of time,” Abu-Ghanem, a urology resident at Sheba Medical Center at Tel HaShomer Hospital in Ramat Gan, Israel, said in her presentation..“…looking at the sum of target lesion measurements, the effect of sunitinib was much more profound in the deferred arm compared [with] the immediate arm.”
The post hoc analysis took into account the number of patients receiving systemic therapy, the overall response rate by RECIST 1.1, the length of drug exposure, and the dose intensity of each arm in the SURTIME study.
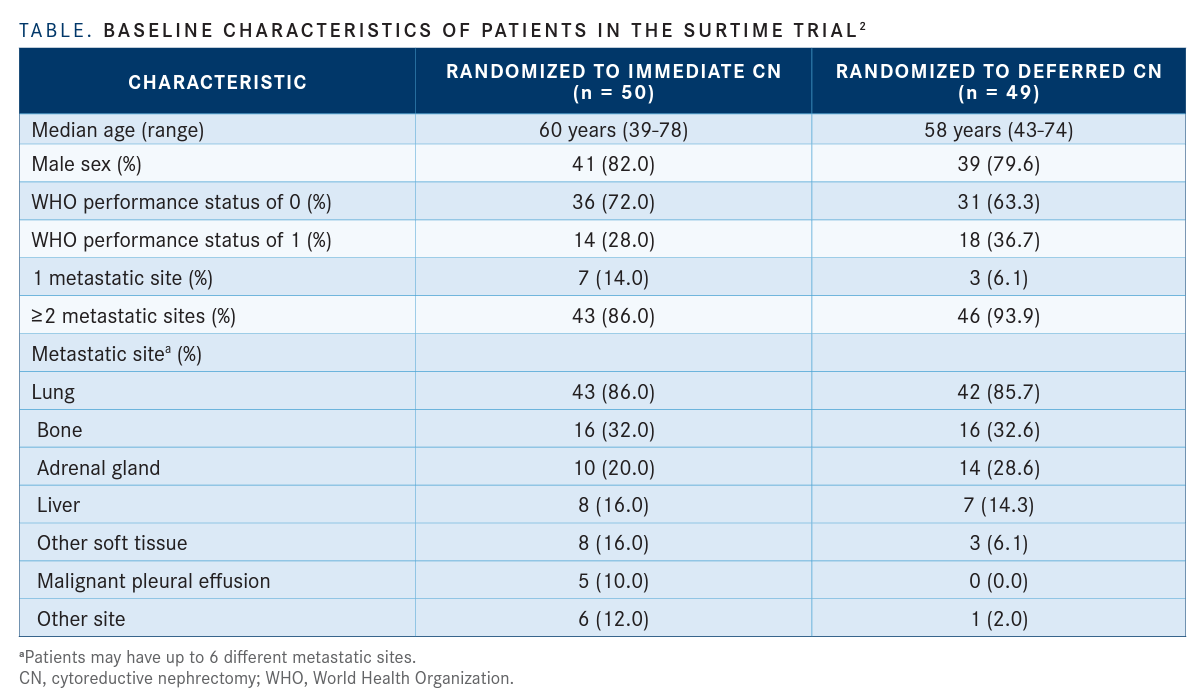
For the original trial, the authors investigated immediate CN followed by sunitinib at 50 mg per day for 4 weeks after surgery in 50 patients with RCC versus 3 cycles of sunitinib followed by CN and then continued therapy for 4 weeks after surgery in 49 patients (TABLE).2
For the intention-to-treat population, the OS hazard ratio favored patients who deferred CN (HR, 0.57; 95% CI, 0.34-0.95; P = .03). This population had a median OS of 32.4 months (95% CI, 14.5-65.3 months) with deferred surgery versus 15.0 months (95% CI, 9.3-29.5 months) with immediate surgery.
OS was a secondary end point, as well as morbidity, overall response in the deferred group, and the effect of CN on early progression in both groups. The primary end point was overall progression-free survival. There was no improvement in the progression-free rate at 28 weeks or in the median progression-free survival.
“With the deferred approach, more patients received sunitinib and OS results were higher. Pretreatment with sunitinib may identify patients with inherent resistance to systemic therapy before planned CN. This evidence complements recent data from randomized clinical trials to inform treatment decisions in patients with primary clear metastatic RCC requiring sunitinib,” the investigators wrote in their abstract.
The FDA approved sunitinib in 2018 for the adjuvant treatment of RCC after data from the multicenter, international, placebo-controlled double-blind S-TRAC trial (NCT00375674).3 The drug was given to 615 patients with a high risk of recurrence after nephrectomy.
References:
1. Abu-Ghanem Y, Van Thienen JV, Blank CU, et al. Differences in the exposure to sunitinib in the immediate and deferred cytoreductive nephrectomy (CN) arms of the randomized controlled trial SURTIME. Poster presented at: 35th Annual European Association of Urology Virtual Congress; July 17-26, 2020. Poster 835. bit.ly/32TpXHQ
2. Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5(2):164-170. doi:10.1001/jamaoncol.2018.5543
3. FDA approves sunitinib malate for adjuvant treatment of renal cell carcinoma. FDA. Updated August 16, 2018. Accessed August 19, 2020. bit.ly/2EitNSm
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen