Petrylak Explores the Use of Maintenance Avelumab in Metastatic Bladder Cancer
During a virtual Targeted Oncology Case-Based Roundtable event, Daniel P. Petrylak, MD, discussed the case of a 66-year-old woman with metastatic bladder cancer.

During a virtual Targeted Oncology Case-Based Roundtable event, Daniel P. Petrylak, MD, professor of Medicine (Medical Oncology) and Urology, Yale School of Medicine, director, Prostate and Genitourinary Medical Oncology, and director, Prostate Cancer Translational Research Group, Yale Cancer Center, discussed the case of a 66-year-old woman with metastatic bladder cancer.
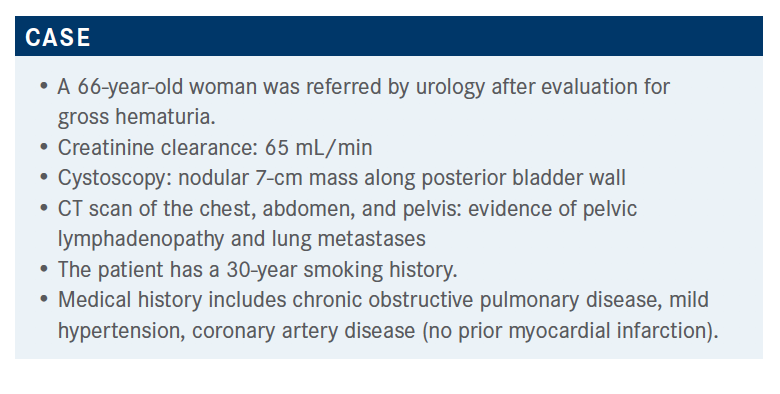
Targeted OncologyTM: What therapies are currently used in bladder cancer?
PETRYLAK: We’ve had considerable experience with MVAC [methotrexate, vinblastine, doxorubicin, and cisplatin], dose-dense MVAC, and gemcitabine/carboplatin, which is the default standard of care for patients who are not eligible to receive cisplatin. For cisplatin-eligible patients, the median survival and confidence limits overlapped. About 22% of patients were alive at 5 years [on dose-dense MVAC].1
With gemcitabine/carboplatin, it was not as good: Median survival was about 9 months and the objective response rate [ORR] was also inferior, 36%. There was a low CR [complete response] rate [with gemcitabine/carboplatin], a higher CR rate with dose-dense MVAC [25%], and a CR rate of 12% with gemcitabine/cisplatin. The ability to potentially render patients long-term survivors is dependent upon their overall performance status, how they’re doing, and whether they can receive cisplatin-based chemotherapy.

Would you consider avelumab (Bavencio) maintenance as a next step? What would influence your decision?
I would agree with this; I think this is the right thing to do. If the patient has no contraindications to immune therapy, avelumab maintenance therapy would be appropriate. Of course, clinical trial trumps all, and we’d want to put a patient on a clinical trial if this were available.
The response is the most important thing: whether the patient responded. The patient could have stable disease as well. The number of cycles in first line is generally 4 to 6. I don’t consider PD-L1 expression in this particular situation; I generally don’t obtain PD-L1 expression unless a patient is platinum eligible and I’m thinking of giving checkpoint inhibitor as front-line therapy. But the comorbidities, particularly immune-related comorbidities, would come into play.
Which trial looks at the data behind avelumab maintenance?
I had the good fortune of being one of the coauthors of the JAVELIN Bladder 100 trial [NCT02603432].2 I helped design this study with Thomas Powles, MD. There was a lot of controversy about this trial because everybody thought that up-front checkpoint therapy combined with chemotherapy was going to be the way to go, and that’s turned out not to be the case.
This trial took patients who were cisplatin eligible, as well as cisplatin ineligible. They received 4 to 6 cycles of first-line chemotherapy, and they had to have a clinical benefit or response to their first-line treatment—CR, partial response [PR], or stable disease [SD]. Seven hundred patients were randomized to receive either avelumab at 10 mg/kg every 2 weeks plus best supportive care [BSC] or BSC alone, and the primary end point was overall survival [OS]. Patients were stratified based upon their response to first-line therapy, metastatic sites, and visceral versus nonvisceral disease. There were 2 populations that were analyzed, all randomized patients and PD-L1–positive patients, and the secondary end points were progression-free survival and ORR, safety and tolerability, and patient-reported outcomes. There was no crossover, and that’s one of the critiques of the trial.2
In the JAVELIN Bladder 100 study, [median] OS for avelumab plus BSC was 21.4 months and for BSC alone was 14.3 months…a 7-month difference. The hazard ratio [HR] was 0.69 [95% CI, 0.56-0.86]. There were 71% of patients alive at 12 months in the experimental arm versus 58% in the control arm, and that declined to 61% versus 44% at 18 months. Clearly there was a survival benefit for giving avelumab earlier.
I think it’s important to look at the different subgroups, and one of the important things is that all patients benefited; the OS HRs based upon PD-L1 expression seemed to be better if you were positive versus negative [HR, 0.56 (95% CI, 0.40-0.78) vs HR, 0.86 (95% CI, 0.62-1.18), respectively]. One of the things about PD-L1 you have to remember is that it’s not only a predictive but a prognostic factor. That may have come into play and be why there was such a major difference. Nonetheless, all-comers benefited… irrespective of creatinine clearance [≥ 60 mL/min: HR, 0.68 (95% CI, 0.50-0.92) vs < 60 mL/min: HR, 0.68 (95% CI, 0.50-0.94)]; best response to first chemotherapy treatment, CR or PR [HR, 0.69 (95% CI, 0.53-0.89)] or SD [HR, 0.70 (95% CI, 0.46-1.05)]; and first-line regimen [gemcitabine/cisplatin: HR, 0.69 (95% CI, 0.51-0.94) vs gemcitabine/carboplatin: HR, 0.66 (95% CI, 0.47-0.91)].
If you think about it for a second, [none of those] should come into play because all that really matters is that the patient responded to the first treatment, no matter what it was. I think one of the strategies for the future is to increase the initial response rate so that more patients are available for maintenance immune therapy.…There are some newer agents now being evaluated that are exciting. The pattern that we saw with checkpoint inhibitors [in JAVELIN Bladder 100] was that patients with visceral disease tended to do a little worse than patients with nonvisceral disease [HR, 0.82 (95% CI, 0.62-1.09) vs HR, 0.54 (95% CI, 0.38-0.76), respectively], and that’s consistent with what we’ve seen with other checkpoint inhibitors.
Does PD-L1 status matter for treatment with avelumab?
OS for both arms was slightly higher in the PD-L1–positive population [avelumab plus BSC: HR, not estimable (NE; 95% CI, 20.3-NE) vs BSC: HR, 17.1 (95% CI, 13.5-23.7)] compared with the overall population [avelumab plus BSC: HR, 21.4 (95% CI, 18.9-26.1) vs BSC: HR, 14.3 (95% CI, 12.9-17.9)].1 That’s the issue we’re talking about: PD-L1 positivity can be a prognostic factor, overall, for survival, and that may be what we’re seeing in the difference in these populations.
What were the response rates and adverse events (AEs) in JAVELIN Bladder 100?
As one would expect, there was a higher ORR in the avelumab arm than in the BSC arm [9.7% (95% CI, 6.8%- 13.3%) vs 1.4% (95% CI, 0.5%-3.3%), respectively]. It was also higher in PD-L1–positive patients [avelumab: 13.8% (95% CI, 9.2%-19.5%) vs BSC: 1.2% (95% CI, 0.1%-4.2%)]. In the overall population, there was a higher CR rate in the avelumab arm, 6%, versus 0.9% the BSC arm; in the PD-L1– positive population it was a little higher as well [9.5% vs 0.6%, respectively]. The overall disease control rate was higher in the avelumab arm versus BSC arm: 41% versus 27.4%, which held true for the PD-L1–positive population as well [43.9% vs 27.8%, respectively].3
In JAVELIN 100, there was the same AE pattern that you would see in patients who are treated with PD-L1 or checkpoint inhibitors for second-line therapy, higher in the avelumab arm than in the BSC arm: classic fatigue [17.7% vs 7%, respectively], pruritus [17.2% vs 1.7%], urinary tract infection [17.2% vs 10.4%]—you expect that with a patient who has had a cystectomy or other urinary tract abnormalities—diarrhea [16.6% vs 4.9%], arthralgias [16.3% vs 5.5%], consistent with what you see with immune therapy. Treatment-related AEs led to discontinuation in about 12% of patients…again, consistent with what we see with checkpoint inhibitors. Death was attributed by the investigator to study treatment in 2 patients in the avelumab arm: 1 was due to sepsis, the other due to an ischemic stroke.3
The [rates of] immune-related AEs were consistent [with other trials, 29.4% vs 7% for the avelumab vs BSC arms]; no grade 4 or 5 events occurred. Steroids were required in 9% of the avelumab-treated patients.3 An interesting subanalysis would be to assess whether the patients who received steroids did better, because that’s a pattern we tend to see with patients who are on checkpoint therapy.
Based upon this phase 3 trial, which was published in the New England Journal of Medicine, the FDA approved avelumab for urothelial carcinoma as maintenance treatment. Patients would receive avelumab every 2 weeks, 4 to 10 weeks after their last chemotherapy treatment.4
How do the National Comprehensive Cancer Network guidelines recommend using avelumab?
For cisplatin-eligible patients, we have gemcitabine/cisplatin followed by avelumab maintenance therapy as one potential treatment and dose-dense MVAC as a front-line therapy, also followed by avelumab maintenance therapy, although a subject of controversy in the design [of JAVELIN 100] was that dose-dense MVAC was not included as one of the agents or regimens in the trial.5 For patients who are cisplatin ineligible, you could use gemcitabine/carboplatin followed by avelumab maintenance therapy or you could give checkpoint inhibitors up front. The advantage of gemcitabine/carboplatin is that it allows people to get their disease under control, and that is crucial, especially since we see some patients who progress rapidly through first-line immunotherapies. I am beginning to favor this approach over the approach of giving atezolizumab or pembrolizumab up front.
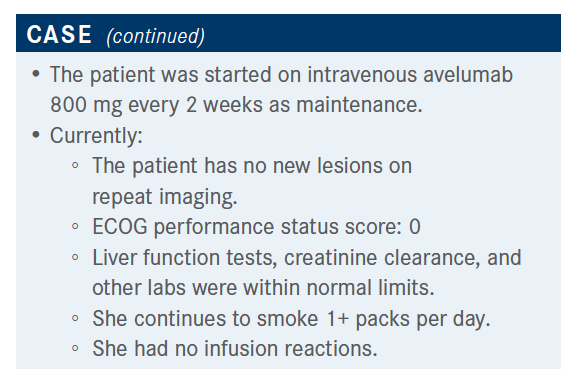
The patient was followed and there were no new lesions on repeat imaging; everything was within normal limits. She had no infusion reactions.
How do you evaluate length of response to multiple lines of therapy?
The response to immunotherapy is much more complex than just PD-L1 expression. There are several other factors it may involve. Perhaps tumor mutational burden can come into play. One of the reasons this trial may be different biologically from other studies is simply that PD-L1 is also an acute phase reactant, and patients’ PD-L1 expression may change after chemotherapy to make them respond at that point. There’s also activation of the immune system. The data with breast cancer looking at neoadjuvant therapy [shows] you’re knocking off [T regulatory cells] with chemotherapy. Does that predispose those patients to becoming more responsive to immune therapy? It’s hard to say.
In comparing JAVELIN 100, a maintenance study, with KEYNOTE-045 [NCT02256436], the pembrolizumab [Keytruda] study in the second line…in KEYONOTE-045, survival was about 10 months for pembrolizumab and 7.2 months for chemotherapy [HR, 0.72 (95% CI, 0.59-0.87)].1 In JAVELIN 100, avelumab plus BSC OS was 21.4 months and BSC alone was 14.3 months [HR, 0.69 (95% CI, 0.56-0.86)]. These were different patient populations and it’s hard to cross-compare trials, but the KEYNOTE-045 OS data are right after patients completed platinum treatment. I think this is a reasonable approach. You would think that you would get better OS with earlier treatment, and now that we have drugs that can extend survival, that’s certainly a better way we can go.
References:
1. Plimack ER. Checkpoint inhibition in metastatic urothelial carcinoma: timing is everything. Presented at: 2020 American Society of Clinical Oncology Virtual Scientific Program; May 29-31, 2020. Abstract LBA1.
2. Powles T, Park SH, Voog E, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. J Clin Oncol. 2020;38(suppl 18):LBA1. doi:10.1200/JCO.2020.38.18_suppl.LBA1
3. Powles T, Park SH, Voog E, at al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. doi:10.1056/NEJMoa2002788
4. FDA approves avelumab for urothelial carcinoma maintenance treatment. News release. FDA. June 30, 2020. Accessed March 15, 2021. https://bit.ly/3jaforD
5. NCCN. NCCN Clinical Practice Guidelines in Oncology. Bladder cancer, version 1.2021. Accessed March 15, 2021. https://bit.ly/3rQRjtr

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More