Multiomics Approach Suggests Positive PFS Correlation With NK Cells in NSCLC
A positive association with progression-free survival in patients with advanced non–small cell lung cancer who were treated with first-line pembrolizumab was observed using a multiomics approach.
A multiomics approach that identified immune circulating cell subsets that were mostly natural killer (NK) cells at baseline, and tumor tissue expression levels of genes, found a positive association with progression-free survival (PFS) in patients with advanced non–small cell lung cancer (NSCLC) who were treated with first-line pembrolizumab (Keytruda).1
Tumor proportion score (TPS) remains a viable predictor of activity, as does tumor mutational burden (TMB), although TMB’s value in predicting overall survival (OS) is debatable. Findings from the PEOPLE trial (NCT03447678), a prospective, monocentric phase 2 study determined that PD-L1 expression of less than 50% might lead to benefit, according to the study published in the Journal for ImmunoTherapy of Cancer.1
From March 21, 2018 to October 7, 2020, 87 patients were screened and 65 were enrolled to receive pembrolizumab at 200 mg administered every 3 weeks and continued for 2 years or 35 cycles.
The primary objective was to identify immune biomarkers associated with PFS and secondary objectives were to detect differences in biomarkers distributed between prestudy and poststudy treatment, objective response rate (ORR), duration of response (DOR), and disease control rate (DCR). The median age was 70 years (range, 47-87) and the majority (68%) were male. PD-L1 TPS expression was 1-49% in 47 patients (73%) and 0 in 18 patients (27%).
Investigators conducted 3 different omics evaluations resulting in 4 different sets of features. These were circulating immune profiling at baseline (bCIP), circulating immune profiling post pembrolizumab (pCIP), gene expression profiling (GEP), and microbiome. At the time of analysis, after a median follow-up of 26.4 months, 51 (78%) patients experienced progression and 49 (70%) died. The median PFS was 2.9 months (95% CI, 1.8-5.8) and the median OS was 12.1 months (95% CI, 9.0-20.2). Investigators reported the ORR was 24.1%, the DCR was 53.4%, and the median DOR was 14.5 months (95% CI, 8.4-24.9).
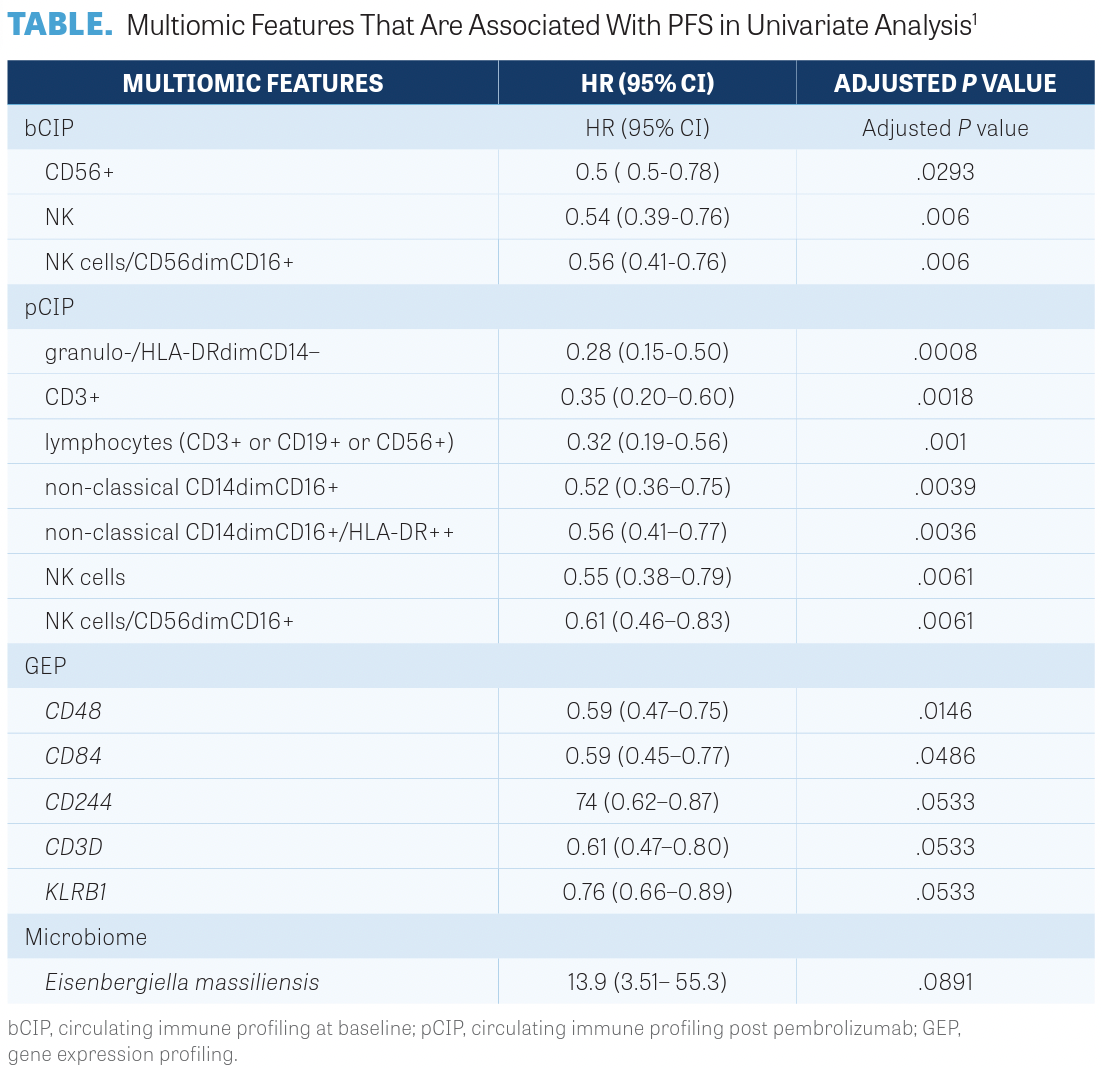
Univariate analysis revealed that each of the 4 data sets correlated with PFS. In the bCIP set, 3 of 36 immune subsets were significantly and positively correlated with PFS as shown in the TABLE.1
Investigators noted that 1 subset at baseline, NK cells/CD56dimCD16-positive, and 3 subsets post pembrolizumab, granulo-/ HLA-DRdimCD14-negative, non-classical CD14dim CD16-positive, and eosinophils CD15-positive CD16-negative, were positively correlated with PFS. The results suggest that NK cells evaluated at baseline may be useful in identifying those patients who may benefit from pembrolizumab.
REFERENCE
1. Lo Russo G, Prelaj A, Dolezal J, et al. PEOPLE (NTC03447678), a phase II trial to test pembrolizumab as first-line treatment in patients with advanced NSCLC with PD-L1 <50%: a multiomics analysis. J Immunother Cancer. 2023;11(6):e006833. doi:10.1136/jitc-2023-006833