Keeping Track of FGFR Inhibitor AEs Leads to Optimal Outcomes
This article explorex some of the more frequently encountered toxicities of FGFR inhibitors and their optimal management.
Continuous focus on fibroblast growth factors (FGFs) remains an important rationale because of their role in normal cellular metabolism as they exert their physiological effects through binding to fibroblast growth factor receptors (FGFRs). This is a complex family of signaling pathways that includes 5 distinct FGFRs (numbered 1 through 5) and 18 identified FGF ligands.1 FGFR receptors have diverse functions including embryogenesis, angiogenesis, wound repair, phosphate metabolism, cellular proliferation, differentiation, migration, and apoptosis.2 Aberrant activation of FGF signaling arising from mutations and amplification of these receptors or gene rearrangements involving the FGFR gene is seen at varying frequencies across a broad range of cancers. Further, it has also been hypothesized that FGF activity contributes to the local immunosuppression within the tumor microenvironment via stimulation of cancer-associated fibroblasts (CAFs).3,4
Consequently, FGFR receptors 1 through 4 have emerged as critical therapeutic targets. FGFR5 lacks a tyrosine kinase domain, so it is not of clinical interest at this time.
Research into this cellular pathway has culminated in the regulatory approval of multiple tyrosine kinase inhibitors including pemigatinib (Pemazyre), infigratinib (Truseltiq), and erdafitinib (Balversa), which target FGFR. Although these therapeutic breakthroughs have increased treatment options for difficult-to-treat cancers such as cholangiocarcinoma and urothelial carcinoma, modulation of the FGF axis poses unique clinical challenges. Unlike other common drug targets, FGFR is rather ubiquitous in normal tissues.
Despite ongoing efforts to develop selective inhibitors targeting specific FGFR subtypes, the close homology between these receptors necessarily results in a certain degree of cross-receptor inhibition, leading to the characteristic toxicities seen with their use. Addressing some of the more frequently encountered toxicities of FGFR inhibitors and their management can lead to positive outcomes.
Hyperphosphatemia
Hyperphosphatemia is a characteristic effect of these agents and arises from inhibition of FGFR1, which has a central role in phosphate metabolism.5 Hyperphosphatemia, unlike other toxicities discussed below, is considered an on-target effect and predictive of anticancer response.6
The significance of this relationship is reflected in the dosing algorithm for erdafitinib, which involves an upward titration of dosing depending on serum phosphate levels. Given the significance of the relationship between tumor response and hyperphosphatemia, management of this adverse event should primarily be focused on strategies to reduce phosphorus levels rather than by dose reduction of the FGFR inhibitor.
TABLE. Special Considerations With Phosphate Binders6
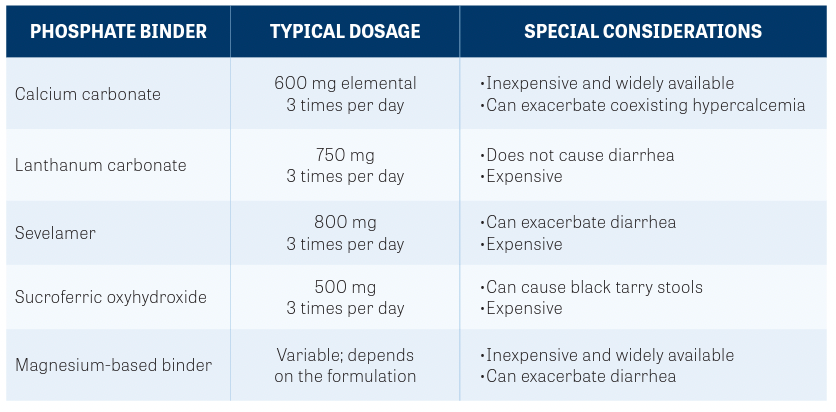
All patients receiving these drugs must be immediately placed on a phosphate- restricted diet and should be provided with educational resources on low-phosphate foods.7 Although most patients can be effectively managed with dietary interventions alone, up to a third may eventually require phosphate binder therapy. Oral phosphate binders are recommended when serum phosphate levels exceed 7 mg/dL.5 The specific type of phosphate binder employed depends on clinical circumstances such as concurrent hypercalcemia or overlapping toxicity such as diarrhea.
Routine monitoring of serum phosphate levels is essential to not only maximize the potential benefit of the FGFR inhibitor, but also to avoid overcorrection and resultant hypophosphatemia. A summary of phosphate binders is provided in the TABLE.6
Diarrhea
Gastrointestinal toxicity, predominantly manifesting as diarrhea, is another commonly encountered adverse event of FGFR inhibition related to the blockade of the FGFR4 receptor. The incidence of diarrhea varies among FGFR inhibitors and is directly correlated with activity against FGFR4.8
Low-grade FGFR inhibitor–associated diarrhea is usually managed with loperamide (Imodium) and supportive care, including intravenous fluids and electrolyte replacement. Higher-grade diarrhea should be managed with dose interruption and resumption at a lower dosage. Fortunately, grade 3 and greater diarrhea is infrequent with these agents, and permanent discontinuation of therapy is rarely needed.
As with other forms of drug-induced diarrhea, it is important to rule out infectious causes before ascribing diarrhea to the drug itself.
Dermatologic Toxicity
FGFR inhibitors are associated with a number of mucocutaneous toxicities. Some of these toxicities are shared with other classes of tyrosine kinase inhibitors (TKIs) such as hand-foot syndrome and stomatitis, which is seen in up to 40% of patients who are administered TKIs.9
Other adverse events are more unusual, such as alopecia (seen in nearly 50% of patients), onycholysis, and nail bed infections, which can develop in approximately 20% of patients.10 FGFR inhibition can also induce dry skin, xerostomia, and dry eye.11
Given the frequency with which these toxicities are reported, it is reasonable to advise patients to start using over-the-counter emollients preemptively and seek immediate attention if they develop such symptoms. When symptoms develop despite emollients, high-potency topical steroids and lidocaine cream can be used in addition to more potent emollients, such as topical urea.
Early dermatology referral should also be considered.12 Onycholysis and paronychia should be managed by keeping the nails as clean and dry as possible and using topical povidone-iodine or vinegar water soaks. Treat refractory cases with oral antibiotics.
Mucosal lubricants and sialagogues such as pilocarpine can be considered when the patient develops dry mouth. Ice chips and good oral hygiene are helpful for minimizing oral mucositis. Once mucositis develops, symptoms can be managed with non–alcohol-containing mouthwash and topical steroids.13
As with other toxicities, FGFR inhibitors should be held for grade 3 severity and then resumed at a lower dose. Alopecia can also be bothersome for many patients and may be managed by topical minoxidil. It is typically reversible with discontinuation of therapy.
Ocular Toxicity
Ocular toxicity is another hallmark of FGFR inhibition seen in approximately one-third of patients and can range from dry eyes to more serious disorders such as central serous retinopathy and retinal detachment.14 Dry eyes can be managed with topical lubricants and may be started prophylactically in patients receiving FGFR inhibitors.15 Close monitoring with periodic ophthalmologic evaluation is key to managing retinal toxicity because it is often reversible with dose interruption when detected early.16 Patients should also be counseled to seek immediate attention in case of vision changes or symptoms such as floaters.
FGFR inhibitors may be resumed if retinal toxicity is promptly reversed after stopping therapy under the supervision of an ophthalmologist. Specific guidance on the frequency of ophthalmologic assessment is available from the manufacturers of individual FGFR inhibitors and should be carefully observed.
Conclusion
FGFR inhibition is associated with several distinctive and often serious toxicities that require meticulous monitoring, early intervention, and potentially, dose adjustment strategies for successful outcomes. As the prevalence of and indications for this class of agents continue to expand, it is increasingly important for practicing oncology clinicians to be familiar with these toxicities and their management.
REFERENCES
1.Yun YR, Won JE, Jeon E, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010:218142. doi:10.4061/2010/218142
2. Belov AA, Mohammadi M. Molecular mechanisms of fibroblast growth factor signaling in
physiology and pathology. Cold Spring Harb Perspect Biol. 2013;5(6):a015958.
doi:10.1101/cshperspect.a015958
3. Katoh M. FGFR inhibitors:effects on cancer cells, tumor microenvironment and whole body
homeostasis (review). Int J Mol Med. 2016;38(1):3-15. doi:10.3892/ijmm.2016.2620
4. Lee HW, Seo HK. Fibroblastgrowth factor inhibitors for treatinglocally advanced/metastatic bladder urothelial carcinomas via dual targeting of tumor-specific oncogenic signaling and the tumor immune microenvironment. Int J Mol Sci. 2021;22(17):9526. doi:10.3390/ijms22179526
5.Kommalapati A, Tella SH, Borad M, Javle M, Mahipal A. FGFRinhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers (Basel). 2021;13(12):2968.
doi:10.3390/cancers13122968
6. Brauer A, Waheed S, Singh T, Maursetter L. Improvement inhyperphosphatemia using phosphateeducation and planning talks. J Ren Nutr. 2019;29(2):156-162. doi:10.1053/j.jrn.2018.06.004
7. Lyou Y, Grivas P, Rosenberg JE, et al. Hyperphosphatemiasecondary to the selective fibroblast growth factor receptor 1-3 inhibitor infigratinib(BGJ398)is associated with antitumor efficacy in fibroblast growth factor receptor 3-altered advanced/metastatic urothelial carcinoma. Eur Urol. 2020;78(6):916-924. doi:10.1016/j.eururo.2020.08.002
8. Camilleri M. Dissectingmolecular mechanisms in bile acid diarrhea. Am J Gastroenterol.
2016;111(3):433-435. doi:10.1038/ajg.2016.23
9. Chakrabarti S, Finnes HD, Mahipal A. Fibroblast growth factor receptor (FGFR) inhibitors in cholangiocarcinoma: current status, insight on resistance mechanisms and toxicity management. Expert Opin Drug Metab Toxicol. 2022;18(1):85-98. doi:10.1080/17425255.2022.2039118
10. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671-684. doi:10.1016/S1470-2045(20)30109-1
11. Loriot Y, Necchi A, Park SH, et al; BLC2001 Study Group. Erdafitinib inlocally advanced or
metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338-348. doi:10.1056/NEJMoa1817323
12. Lacouture ME, Sibaud V, Anadkat MJ, et al. Dermatologicadverse events associated with
selective fibroblast growth factor receptor inhibitors: overview, prevention, and management guidelines. Oncologist. 2021;26(2):e316-e326. doi:10.1002/onco.13552
13. Bensinger W, Schubert M, Ang KK, et al. NCCNtask force report. prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(suppl 1):S1-S21; quiz S22-S24.
14. Mahipal A, Tella SH, Kommalapati A, Yu J, Kim R. Prevention and treatment of FGFR inhibitor-associated toxicities. Crit Rev Oncol Hematol. 2020;155:103091. doi:10.1016/j.critrevonc.2020.103091
15. Lee PC, Hendifar A, Osipov A, Cho M, Li D, Gong J. Targeting thefibroblast growth factor
receptor(FGFR) in advanced cholangiocarcinoma: clinical trial progress and future
considerations. Cancers (Basel). 2021;13(7):1706. doi:10.3390/cancers13071706
16. Francis JH, Harding JJ, Schram AM,et al. Clinical andmorphologic characteristics of fibroblast growth factor receptor inhibitor-associated retinopathy. JAMA Ophthalmol. 2021;139(10):1126-1130. doi:10.1001/jamaophthalmol.2021.3331