Quizartinib Improves OS in FLT3-ITD+ AML
The phase 3 QuANTUM-First trial showed that quizartinib plus chemotherapy with or without allogeneic hematopoietic stem cell transplant improved overall survival in patients with acute myeloid leukemia with a FLT3-ITD mutation.
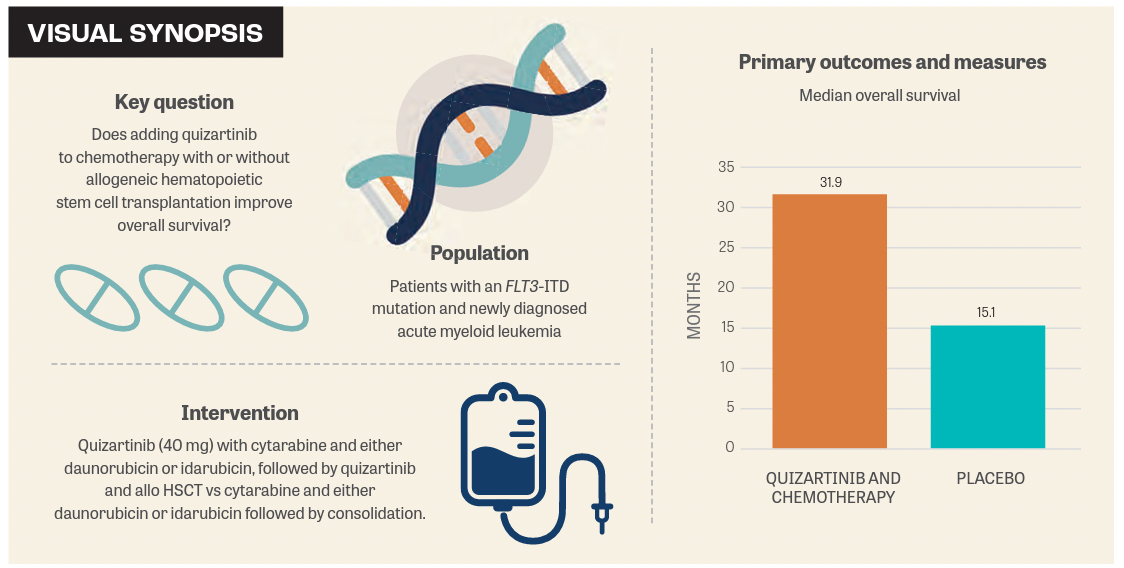
A clinically meaningful and statistically significant improvement in overall survival (OS) was achieved with the addition of quizartinib to chemotherapy with or without allogeneic hematopoietic stem cell transplant (allo HSCT) compared with chemotherapy and placebo for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) with an FLT3 internal tandem duplication (FLT3-ITD) mutation.1
Findings from the phase 3 QuANTUM-First trial (NCT02668653) were presented at the European Society for Blood and Marrow Transplantation (EBMT) 49th Annual Meeting. A total of 539 patients were included in the study, 268 of whom were treated with the quizartinib combination and 271 of whom received chemotherapy and placebo.
“Treatment with quizartinib showed an overall better survival with a median survival of almost 32 months compared [with] the placebo-treated patients who had a median [OS] of 15 months,” said Radovan Vrhovac, MD, University of Zagreb School of Medicine, Croatia, in a presentation of the data at the EBMT meeting.
Quizartinib is a highly potent and selective second-generation type 2 FLT3 inhibitor that has shown a signal of clinical efficacy along with a manageable safety profile in previous studies.
In the experimental arm of QuANTUM-First, patients received up to 2 cycles of quizartinib 40 mg with cytarabine and either daunorubicin or idarubicin for up to 4 cycles, as well as cytarabine consolidation followed by quizartinib and allo HSCT.
Patients in the control arm were given 2 cycles of cytarabine and either daunorubicin or idarubicin followed by placebo, then 4 cycles of consolidation therapy. In the trial, treatment continued for up to 36 cycles.1
Those enrolled in the study were aged 18 to 75 years with newly diagnosed FLT3-ITD–positive AML. Among all patients, baseline characteristics showed that the median age of those enrolled was 56 years (range, 20-75), most patients were female (54.5%), had an ECOG performance status of 1 (50.1%), and had mutated NPM1 (52.3%).
OS, overall survival, all-HSCT, allogeneic hematopoietic stem cell transplant. Schlenk RF, Montesinos P, Romero-Aguilar A, et al. Impact of allo-HCT in fi rst complete remission (Cr1) in addition to FLT3 inhibition with quizartinib in AML with FLT3-ITD: results from the QuANTUM-First trial. Presented at: EBMT 49th Annual Meeting; April 23-26, 2023; Paris, France. OS01-04.
Patients who achieved complete responses (CRs) (n = 297) had a median age of 56 years (range, 20-75) compared with 51 years (range, 20-70) for patients who underwent allo HSCT (n = 157). Patients who achieved a CR and those being transplanted also had a higher proportion of NPM1 mutations (65.7% and 64.3%, respectively).
The primary end point of the study was to assess OS. The secondary end points were event-free survival, CR rate, composite CR, composite CR with FLT3-ITD mutation with minimal residual disease (MRD), and the number of patients with treatment- emergent adverse events.
The median time from enrollment in the study to transplantation was roughly 3.5 months (range, 0.6-11.2) in the quizartinib arm (n = 84) and 3.3 months (range, 0.8-11.6) in the placebo arm.
For both the quizartinib and placebo arms, most patients received an ablative regimen (72.6% and 61.6%) from an unrelated donor (47.6% and 52.1%).
In most cases, these were fully matched (72.6% and 69.9%), and the source of stem cells was mostly peripheral blood (78.6% and 84.9%).
The median follow-up was 39.2 months (95% CI, 37.2-41.5 months) in the quizartinib arm and 39.2% (95% CI, 36.3-41.2 months) in the placebo arm.
For the primary end point, the median OS observed with quizartinib and chemotherapy was 31.9 months (95% CI, 21.0 months-not evaluable [NE]) vs 15.1 months (95% CI, 13.2-26.2) with placebo (HR, 0.78; 95% CI, 0.62-0.98; P = .032).
Treatment with quizartinib and allo HSCT in first remission had a prognostic impact on OS at 0.77 (95% CI, 0.61-0.97; P = .0284). HR was .42 (95% CI, 0.301-0.597) with a significant P value of less than .0001.
“These plots are demonstrating the survival of patients according to the randomization arm and whether they were transplanted or not.… Patients randomized on quizartinib did better than their counterparts receiving placebo, regardless of [whether] they were transplanted or just continued consolidation therapy and maintenance,” Vrhovac said.
For the final post hoc analysis done on this population of patients, investigators looked at the MRD status before transplantation in patients who were transplanted. The MRD status was performed before transplantation.
Findings showed that patients on quizartinib did better than patients on placebo, regardless of their MRD status. This was especially true for the MRD-positive patients.
“This study has shown that the addition of quizartinib provides a clinically meaningful and statistically significant improvement in [OS] compared with standard induction consolidation therapy alone. These post hoc analyses show that patients who underwent stem cell transplantation in CR1 had a longer survival and that irrespective of our stem cell transplantation on CR1, longer survival was observed in those treated with quizartinib vs placebo. Finally, irrespective of the pre–allo HSCT MRD status, longer survival was observed in those treated with quizartinib vs placebo,” concluded Vrhovac.
REFERENCE
1. Schlenk RF, Montesinos P, Romero-Aguilar A, et al. Impact of allo-HCT in first complete remission (Cr1) in addition to FLT3 inhibition with quizartinib in AML with FLT3-ITD: results from the QuANTUM-First trial. Presented at: EBMT 49th Annual Meeting; April 23-26, 2023; Paris, France. OS01-04.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More