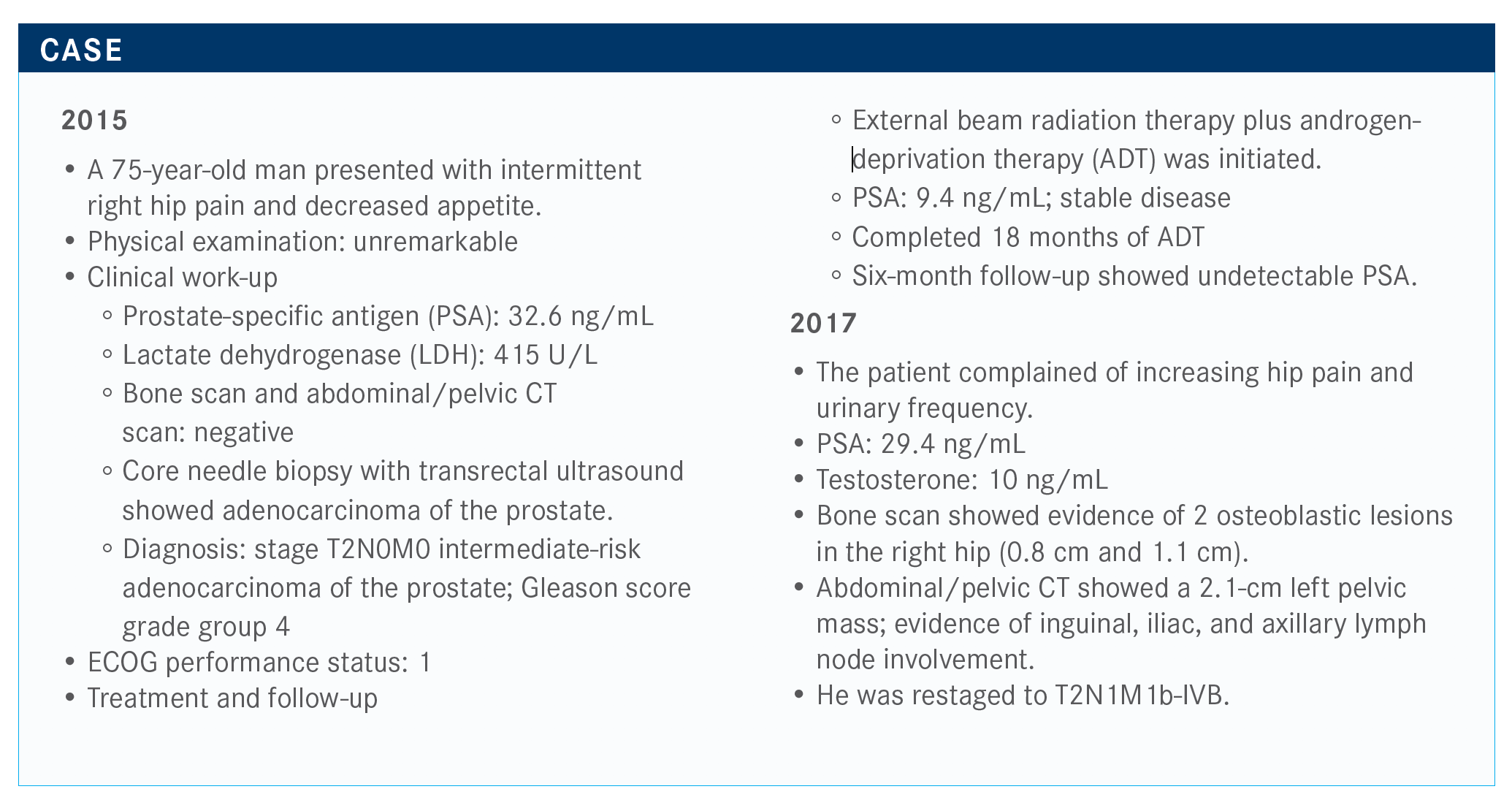
Morgans Addresses Neuropathic Toxicity in Second-Line Metastatic CRPC
Alicia K. Morgans, MD, MPH, discussed the case of a 75-year-old male with metastatic castration-resistant prostate cancer.
Alicia K. Morgans, MD, MPH

During a Case Based Peer Perspective event, Alicia K. Morgans, MD, MPH, associate professor of Medicine, Hematology and Oncology, at the Feinberg School of Medicine, Northwestern University in Chicago, IL, discussed the case of a 75-year-old male with metastatic castration-resistant prostate cancer (mCRPC).
Targeted Oncology™: What are potential treatment options for the second-line setting in patients with metastatic castration-resistant prostate cancer (mCRPC)?
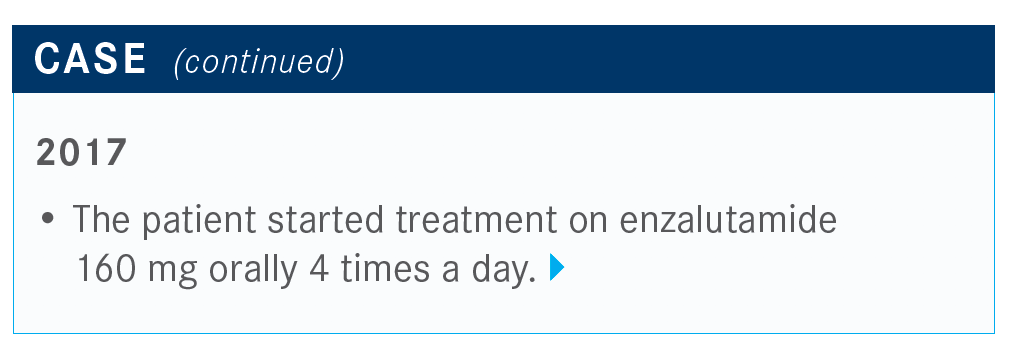
MORGANS: This patient was started on enzalutamide [Xtandi] and had progression after 8 months; he was started on docetaxel at that time. He received 4 cycles but had neuropathy that resulted in the patient and provider stopping the treatment.
The patient had a rising PSA level and required treatment. We know that we have multiple treatment options in this second-line setting for mCRPC.
We have to consider what these patients were treated with prior to seeing us. This includes assessing the fitness of the patient and the patient’s goals and preferences. We now know that this patient was treated with enzalutamide. We also know that the options are abiraterone acetate [Zytiga], cabazitaxel [Jevtana], and docetaxel.
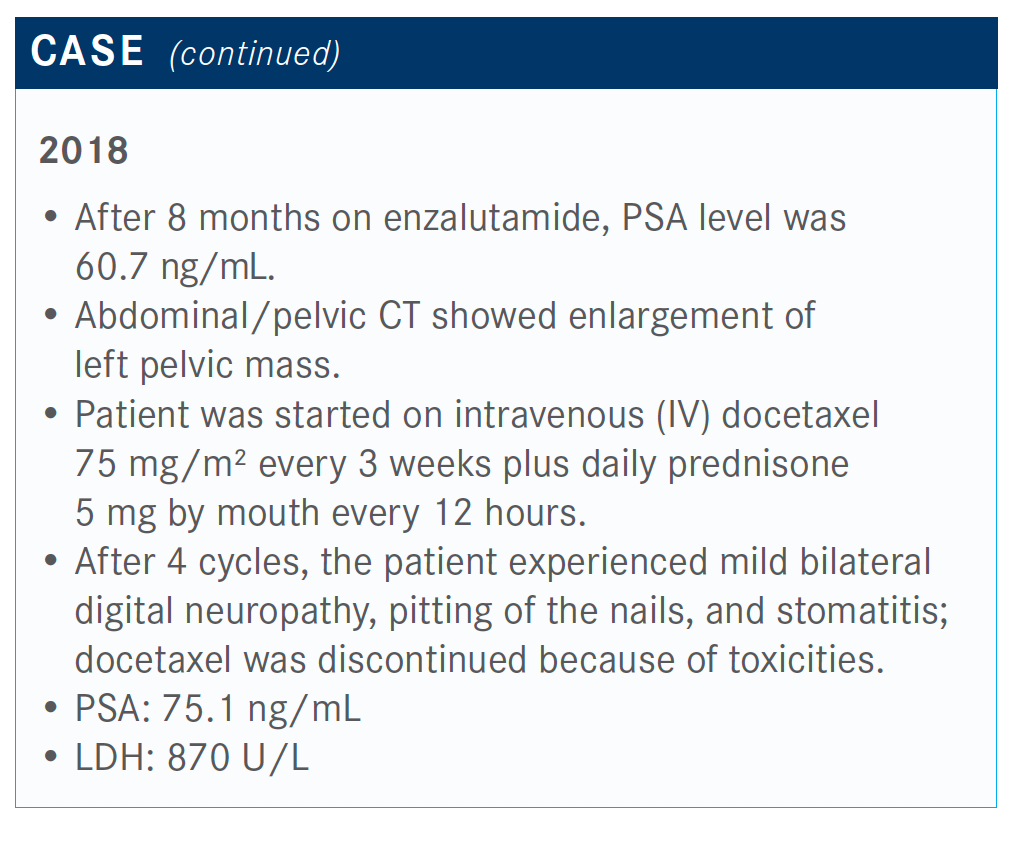
After 4 cycles, the patient experienced neuropathy and a rising PSA level after less than 12 months on treatment.
Would you give cabazitaxel to a patient after experiencing neuropathy from docetaxel?
That would be a good treatment option.
We have data from a study in patients with mCRPC called FIRSTANA [NCT01308567].1 Patients were randomized in the first-line setting in mCRPC to receive either cabazitaxel 25 mg/m2, cabazitaxel 20 mg/m2, or docetaxel 75 mg/m2. For cabazitaxel, the 20-mg/m2 dose replaced the 25-mg/m2 dose in the NCCN [National Comprehensive Cancer Network] guidelines in the United States.2
Although none of these agents demonstrated superiority in terms of disease control or efficacy, there was a slightly higher response observed in the cabazitaxel 25-mg/m2 dose versus the 20-mg/m2 dose. Fewer adverse events [AEs] such as neuropathy, neutropenia, and neutropenic fever were observed in patients who received 20 mg/m2 versus docetaxel, which was a little surprising to me.
The study came out just a few years after cabazitaxel was approved.3 In most cases, I start at the 20-mg/m2 dose rather than the 25 mg/m2. We can give this in patients who have had complications with docetaxel. It’s on a cycle-by-cycle basis, but it seems reasonable based on these data and on personal experience. I thought this was an interesting postapproval study that was required of the organization to enlighten us about different doses.
There is also the phase 4 CARD study [NCT02485691], which was shared at the ESMO [European Society of Medical Oncology] Congress last year.4 These patients had already progressed on their AR [androgen receptor]–targeted agent and docetaxel. This is a European study, so patients had received an AR-targeted agent in the metastatic castration-resistant setting as well as chemotherapy in the metastatic castration-resistant setting. They were not patients who received AR-targeted agents in the hormone-sensitive setting.
Patients were randomized for their next treatment to cabazitaxel at the full 25-mg/m2 dose with Neulasta [pegfilgrastim versus either abiraterone or enzalutamide, depending on which agent that they had not previously received and progressed on in an earlier phase of the disease. Radiographic progression-free survival [rPFS] was the primary end point, overall survival was a secondary end point, as well as quality-of-life concerns including pain and PSA responses.
The baseline characteristics suggested that there were more patients who were older in the cabazitaxel arm. The investigators reported that patients in the AR-targeted arm experienced pain more frequently, but overall, both arms were fairly well matched. This was a small study to begin with, but they matched well overall
Please explain the efficacy of the CARD trial.
We saw that the rPFS was significantly better for patients who received cabazitaxel versus those treated with either abiraterone or enzalutamide. The median rPFS for the cabazitaxel arm was 8.0 months versus the abiraterone or enzalutamide arm at 3.7 months [HR, 0.54; 95% CI, 0.40-0.73; P <.0001]. Subgroup analysis revealed that most subgroups benefited from an rPFS standpoint.
The overall survival data were also presented at ESMO, and this is why I think it was considered one of the presidential symposia. Patients treated with cabazitaxel had a significant improvement in mortality as compared with patients who received either abiraterone or enzalutamide.
There was a 46% reduction in mortality when patients were treated with cabazitaxel compared with the other 2 regimens. Although this is a higher dose than what I normally prescribe, these patients were still able to tolerate it and had an improved survival.
Interestingly, there were significantly more PSA responses reported with treatment with cabazitaxel versus an AR-targeted agent, and significantly more tumor responses [reported] by [the] RECIST [trial]. When investigators measured the tumor size, it seemed to have decreased in size, and a much better pain response was observed. This was determined by using the percentage of the patients who had an improvement in their pain score that was maintained and clinically meaningful over time. Better pain control with cabazitaxel was reported as well. Investigators also observed a longer time to symptomatic skeletal events in the cabazitaxel arm versus the AR-targeted agent.
What’s important to note is when looking at chemotherapy versus AR-targeted agents, there were more AEs that led to treatment discontinuation, with slightly more discontinuations in the cabazitaxel arm. What surprised me and a fair number of people was that there were more AEs leading to death in those patients treated with the AR-targeted agents than with the chemotherapy. This suggests more mortality-related AEs associated with the AR-targeted agents.
Health-related quality of life seemed to be maintained, probably because the disease was better controlled. This was just presented at the 2020 Genitourinary Cancers Symposium. Patients had better pain control and certainly better prostate-specific concerns with cabazitaxel than with the AR-targeted agents, at least through cycle 5 or so.⁵
What other presentations at the 2019 ESMO Congress were noteworthy?
The other study that I think was groundbreaking at ESMO was a phase 3 trial that targeted DNA repair defects.6 PROfound [NCT02987543] evaluated patients with mCRPC who had also progressed on the AR-targeted agents as well as chemotherapy. They were randomized to receive olaparib [Lynparza] or the second AR-targeted agent. All patients had to have DNA repair defects in their tissue to get into this trial. This was an ambitious study to carry out in a population with prostate cancer. The investigators were able to complete it and do a nice phase 3 trial and present it.
When the 2 arms were compared, the rate of AEs was higher in the olaparib arm; those included cytopenias, some nausea, and other complications. Those are some of the common AEs. Importantly, the treatment duration was significantly longer for patients treated with olaparib than AR-targeted agents.
Reviewing the disease response, rPFS as determined by a blinded independent reviewer was significantly longer for patients treated with olaparib compared with patients who received that second AR-targeted agent. Importantly, patients who received that second AR-targeted agent did not have any restrictions on how long they had to have been on that agent in the first place.
This differs from the CARD study, in which patients had to be on treatment for 12 months or less with a response to an AR-targeted agent. These patients, in contrast, could have been on for however long they were responding; they just had to have received treatment in the past. We see at 3.5 months, 50% of patients treated with that second AR-targeted agent are falling off for radiographic progression, which demonstrates that it’s not an effective treatment. In any event, olapaolaparib seemed to be significantly more effective in terms of rPFS.
Looking at the confirmed overall response rate in cohort A, which included patients with BRCA1/2 and ATM mutations, there was a significant improvement in objective response rate, 33% versus 2.3% [odds ratio, 20.86; 95% CI, 4.18-379.18; P <.0001].
Reviewing the data for cohort B, which included patients with DNA repair-defect mutations and not just BRCA1/2 or ATM mutations, the investigators reported an improvement in rPFS for this selected patient population.

What did the patient end up receiving?
In this particular case, the patient received cabazitaxel. In a pandemic, I think it’s especially important to try to prevent our patients from being hospitalized for neutropenic fever.
References:
1. Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017;35(28):31893197. doi:10.1200/ JCO.2016.72.1068
2. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 1.2020. Accessed May 12, 2020. https://www.nccn.org/professionals/physician_gls/pdf/ prostate.pdf
3. FDA approves lower dose of cabazitaxel for prostate cancer. Updated September 14, 2017. Accessed May 12, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lower-dose-cabazitaxel-prostate-cancer
4. deWit R, Kramer G, Eymard J, et al. CARD: randomized, open-label study of cabazitaxel (cbz) vs abiraterone (abi) or enzalutamide (enz) in metastatic castration-resistant prostate cancer (mcrpc). Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394
5. Fizazi K, Kramer G, Eymard J-C, et al. Pain response and health-related quality of life (HRQL) analysis in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving cabazitaxel (CBZ) versus abiraterone or enzalutamide in the CARD study. J Clin Oncol. 2020;38(suppl 6):16. doi:10.1200/JCO.2020.38.6_suppl.16
6. Hussain M. PROFOUND: phase 3 study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mcrpc) with homologous recombination repair (hrr) gene alterations. Ann Oncol. 2019;30(suppl 5):v851- v934. doi:10.1093/annonc/mdz394

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More