Hill Compares Toxicity Profiles of Current Treatment Regimens in Relapsed CLL
During a Case Based Peer Perspectives event Brian T. Hill, MD, PhD, discussed the case of a 53-year-old patients with chronic lymphocytic leukemia.
Brian T. Hill, MD, PhD

During a Case Based Peer Perspectives event Brian T. Hill, MD, PhD, director, Lymphoid Malignancy Program, Cleveland Clinic discussed the case of a 53-year-old patients with chronic lymphocytic leukemia (CLL).
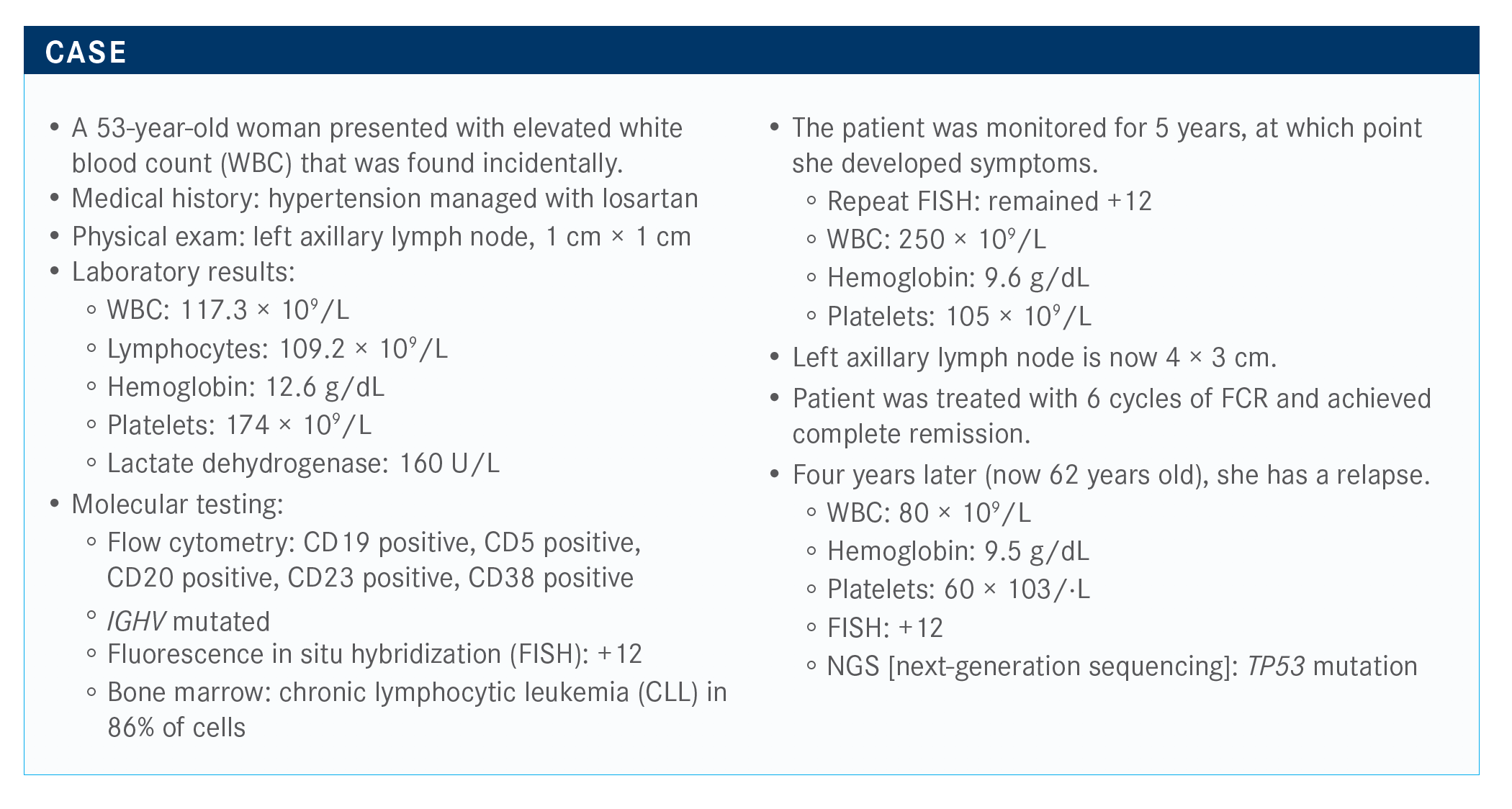
Targeted Oncology™: What regimen are you most likely to recommend for this patient with relapsed CLL and a TP53 mutation?
HILL: For this patient, we can consider ibrutinib [Imbruvica] or acalabrutinib [Calquence], maybe venetoclax [Venclexta] and rituximab [Rituxan]. The NCCN [National Comprehensive Cancer Network] guidelines suggest the preferred regimens are acalabrutinib, with or without obinutuzumab [Gazyva], ibrutinib, and venetoclax with obinutuzumab.1
How do the toxicities between ibrutinib and acalabrutinib compare?
Both agents are active, but there are unique toxicities associated with acalabrutinib, like headaches. Although it sounds like a minor toxicity, it can be significant for some patients, [especially] if they experience a headache every day.
Prior to the approval of ibrutinib, what was the standard of care?
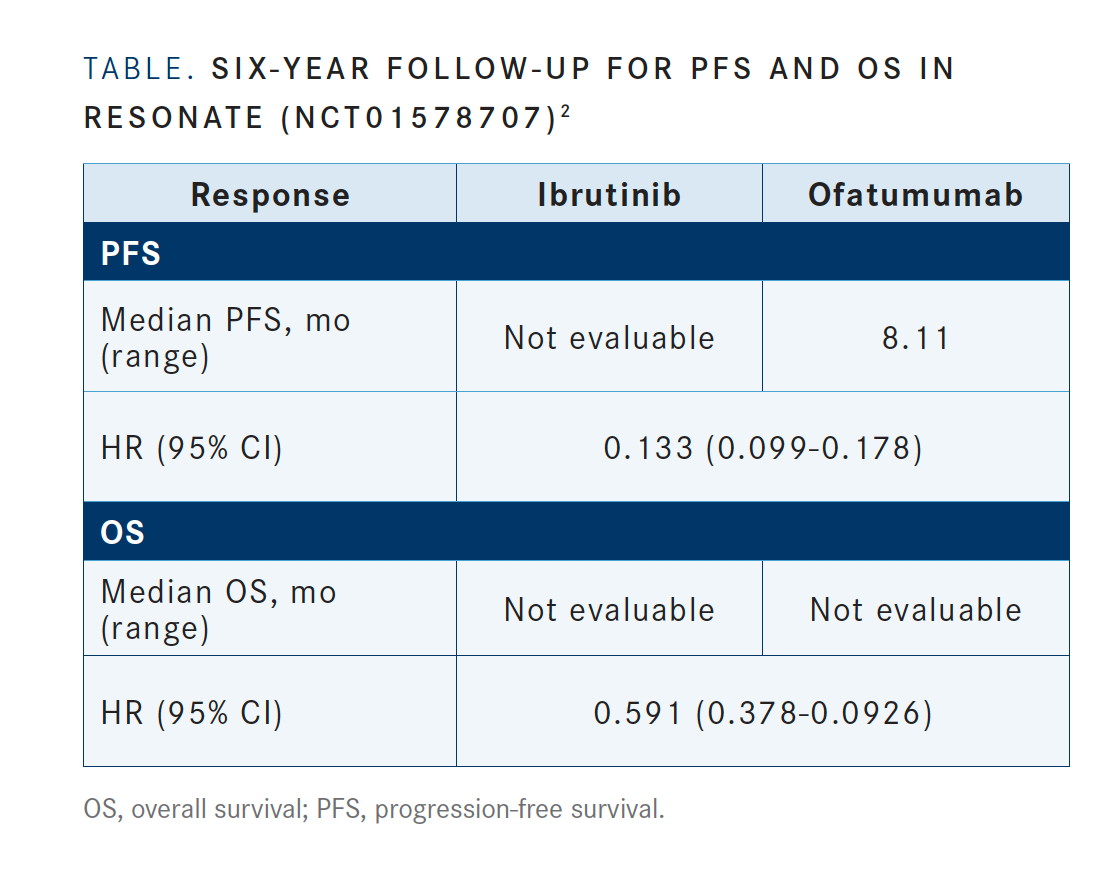
The results from RESONATE [NCT01578707]—which compared ofatumumab [Arzerra], the standard of care, with ibrutinib in patients with previously treated CLL—demonstrated a progression-free survival [PFS] advantage of ibrutinib over ofatumumab after a 6-year follow-up [TABLE; HR, 0.133 95% CI, 0.099-0.178].2 The overall survival [OS] for the 2 arms favored ibrutinib as well; HR, 0.591, 95% CI, 0.378-0.926]. These results demonstrate the importance of giving the best treatment in the relapsed setting. You want to go with the best treatment that you have.
Reviewing the adverse effects greater than or equal to grade 3 demonstrates that over time, infections tend to dissipate rather than increase in number.
In the relapsed setting, please compare acalabrutinib to idelalisib (Zydelig) or bendamustine.
The phase 3 randomized trial in the relapsed setting ASCEND [NCT02970318] evaluated the 1:1 randomization of patients treated with the BTK [Bruton tyrosine kinase] inhibitor acalabrutinib versus investigator’s discretion therapy. Patients either received acalabrutinib or the PI3K inhibitor idelalisib and rituximab or bendamustine and rituximab.3
In this setting, the median PFS for acalabrutinib has not been reached yet, and the median PFS for idelalisib and bendamustine was 16.5 months. One-year PFS rate was reported at 88% for acalabrutinib compared with 68% in patients who received idelalisib and bendamustine (HR, 0.31; 95% CI, 0.20-0.49; P <.0001).
Reviewing the subgroup analysis toxicity data, they still favor the BTK inhibitor in all subgroups. [Regarding] adverse events and safety, we haven’t discussed the toxicities for idelalisib and rituximab, but there can be a fair amount of diarrhea. In the ASCEND trial, the investigators reported that 47% of patients experienced diarrhea with idelalisib, [whereas] diarrhea was much lower with the other agents. Regarding headache, 23% of patients experienced headache in the acalabrutinib arm. It was reported as grade 3 or greater.
When choosing a BTK inhibitor, what findings from the trial for the treatment are most influential?
I think most clinicians are concerned about duration of response, with length of follow-up being less important.
In the relapsed setting of CLL, the MURANO study [NCT02005471] evaluated patients who received venetoclax with rituximab or bendamustine with rituximab. Most of these patients had received prior chemoimmunotherapy, but none had received a prior BTK inhibitor.4
In the study, the 4-year PFS rate for venetoclax was remarkably better for bendamustine [75.5% vs 68.0%; HR, 0.19; P <.0001]. This demonstrates that bendamustine and rituximab in the relapsed setting should be avoided, in my opinion, because you get such a dramatically improved PFS by using venetoclax over chemotherapy. [Further], there is a remarkable improvement in the [OS for venetoclax] compared with chemoimmunotherapy. The MURANO trial is important because in contrast to some of the other studies, where the comparator was ofatumumab or drugs we don’t use, bendamustine and rituximab is a standard regimen.
The preplanned interim analysis of subgroups showed that patients with or without a 17p deletion had equal benefit. Across all subgroups, there was a PFS advantage to venetoclax. Objective response rates also favored venetoclax, 92% versus 72%. The complete remission rate was higher for the group receiving venetoclax compared with the group receiving bendamustine. Negative MRD [minimal residual disease] rates were significantly higher in the venetoclax arm, at 83%. That’s according to peripheral blood, which is why the numbers are so high.
Reviewing the grade 3/4 treatment-related adverse effects indicates that patients who received venetoclax had a higher incidence of neutropenia compared with bendamustine [58.8% vs 39.9%, respectively]. Interestingly, if you look at the incidence of febrile neutropenia, patients in the venetoclax [arm] experienced it less frequently than [those who received] bendamustine [3.6% vs 9.6%, respectively]. I think that there is some unique toxicity associated with venetoclax that causes neutropenia, but it doesn’t necessarily lead to neutropenic fever.
What are the treatment options for patients with relapsed CLL who are not candidates for chemotherapy?
The phase 3 IDELA study [NCT02136511] evaluated idelalisib and rituximab compared with rituximab and placebo.5 These drugs are active. The PFS of idelalisib and rituximab was remarkably better than rituximab alone [19.4 months (95% CI, 12.3–not reached) vs 6.5 months (95% CI, 4.0-7.3)]. In addition, OS also favored idelalisib and rituximab over rituximab alone [40.6 months (95% CI, 28.5-57.3) vs 34.6 months (95% CI, 16.0–not reached)].
Another option is duvelisib [Copiktra]. The phase 3 DUO study [NCT02004522] compared duvelisib with the comparator ofatumumab.6 The median PFS rate favored duvelisib over ofatumumab, although it doesn’t look quite as striking as idelalisib data [13.3 months (95% CI, 12.1-16.8) compared with 9.9 months (95% CI, 9.2-11.3); P <.0001].
References:
1. NCCN. Clinical Practice Guidelines in Oncology. Chronic lymphocytic leukemia/ small lymphocytic lymphoma, version 4.2020. Accessed May 19, 2020. nccn.org/ professionals/physician_gls/pdf/cll.pdf
2. Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031-2042. doi:10.1182/ blood-2018-08-870238
3. Ghia P, Pluta A, Wach M, et al. Acalabrutinib vs rituximab plus idelalisib (idr) or bendamustine (br) by investigator choice in relapsed/refractory (rr) chronic lymphocytic leukemia: phase 3 ASCEND study. Hematol Oncol. 2019;37(S2):86-87. doi:10.1002/ hon.54_2629
4. Seymore JF, Kipps TJ, Eichors BF, et al. Venetoclax plus rituximab is superior to bendamustine plus rituximab in patients with relapsed/ refractory chronic lymphocytic leukemia - results from pre-planned interim analysis of the randomized phase 3 MURANO study. Blood. 2017;130(suppl 1):LBA-2. doi:10.1182/blood.V130.Suppl_1. LBA-2.LBA-2
5. Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391-1402. doi:10.1200/JCO.18.01460
6. Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446-2455. doi:10.1182/ blood-2018-05-850461