Liquid Biopsies Can Assist Therapy Selection in Multistage Colon Cancer
To move the treatment of colon cancer forward, investigators are identifying better biomarkers for response to adjuvant chemotherapy and as early indicators of chemotherapy success, said Jeanne Tie, MD, MBChB, to an audience at the ASCO: Medical Crossfire®: How to Use Liquid Biopsies in Oncology Care hosted by Physicians’ Education Resource®, LLC.
Jeanne Tie, MD, MBChB

Jeanne Tie, MD, MBChB
To move the treatment of colon cancer forward, investigators are identifying better biomarkers for response to adjuvant chemotherapy and as early indicators of chemotherapy success, said Jeanne Tie, MD, MBChB, to an audience at the ASCO: Medical Crossfire®: How to Use Liquid Biopsies in Oncology Care hosted by Physicians’ Education Resource®, LLC.1
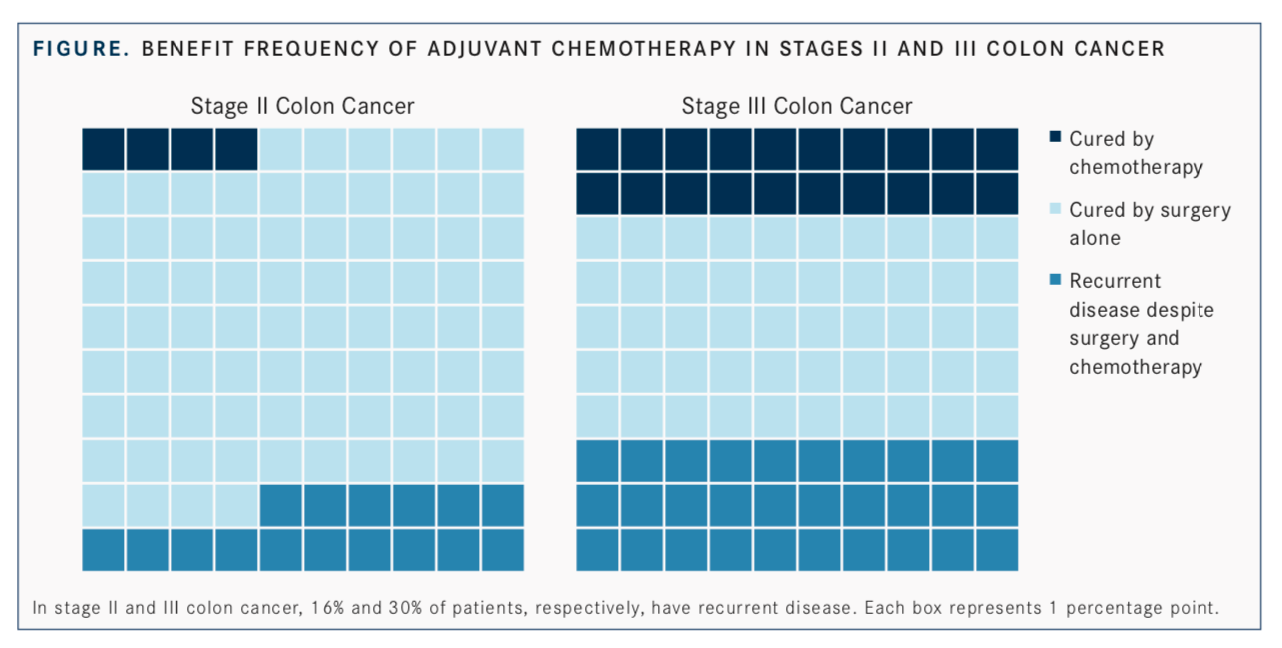
Early-stage disease presents a challenge in the clinic because despite best efforts, identification of patients at high risk of disease recurrence is not always successful. Tie, the lower gastrointestinal medical and trials lead at the Peter MacCallum Cancer Centre and an associate professor at the University of Melbourne in Australia, said that only 4% of patients with stage II disease will benefit or be cured with adjuvant chemotherapy; similarly, only 1 in 5 patients with stage III disease will see this same benefit. The remainder of patients will be cured by surgery alone or have recurrent disease after adjuvant chemotherapy (FIGURE).
One study prospectively evaluated plasma samples from 230 patients with stage II colon cancer using a parallel sequencingbased assay for 15 gene sets known to be mutated in colon cancer to identify patient-specific somatic mutations that would inform the circulating tumor (ct)DNA analysis. The majority of patients who did not receive chemotherapy (79.0%) and were ctDNA positive at 4 to 6 weeks following surgery had recurrent disease versus only 9.8% of those who were ctDNA negative (HR, 18; 95% CI, 7.9-40.0;P<.001). The estimated 3-year recurrence-free survival (RFS) rates for these patients were 0% and 90%, respectively.2
Guided Approach to Adjuvant Therapy
When patients were divided into high- and low-risk groups, the prognostic impact of ctDNA remained highly significant, said Tie. The clinically low-risk group was defined as those with no poor prognostic features. “Of particular clinical relevance is that postoperative ctDNA testing identified those patients at high risk of recurrence in the clinically low-risk group for whom we would not typically be offering chemotherapy,” she said.The next potential application for ctDNA, said Tie, is in tracking the effectiveness of chemotherapy by microscopic tumor burden in real time. In patients with stage III colon cancer who were planned for adjuvant chemotherapy, one study found that rates of 2-year RFS were worse in patients who were ctDNA positive following surgery compared with those who had negative results (47% vs 84%; HR, 3.8; 95% CI, 2.4-21.0;P<.001). Reanalysis of plasma in the same group of patients revealed that the difference in 2-year RFS between those who had positive and negative results following 6 months of chemo- therapy was markedly worse, at 27% versus 82%, respectively (HR, 6.8; 95% CI, 11-157).3
Tie said these data open a window of opportunity for intervention in patients with these features, particularly those in high-risk populations. She suggested challenging ctDNA-positive patients with novel therapies in combination with chemotherapy in the immediate postoperative setting or introducing treatment after chemotherapy has failed to eradicate the ctDNA.
Using Assays in Metastatic Colon Cancer
Several ongoing clinical trials are examining the ctDNA-guided approach to adjuvant chemotherapy. The phase II DYNAMIC study is looking at a ctDNA- guided approach to adjuvant therapy in patients with stage II disease (ACTRN12615000381583). The DYNAMIC-III trial in stage III disease will use ctDNA results to decide between either a de-escalation or escalation adjuvant treatment strategy (ACTRN12617001566325).Frequently, plasmaRASgenotyping is utilized to decide whether to use antiepidermal growth factor receptor (EGFR) therapy in metastatic colon cancer, such as cetuximab (Erbitux) or panitumumab (Vectibix). “Unlike in lung cancer, where you are looking for a mutation to target, we are actually looking for a mutation to withhold treatment from,” Tie said.
She then reviewedRASgenotyping between plasma and tissue samples in several recent studies and concluded that the concordance between the 2 methods was high, around 90%.1
Managing Emerging Resistance
Tie went on to explain that high overall agreement in treatment outcomes exists between patients who have tissue or plasmaRAStesting. A prospective-retrospective study of patients treated with an anti-EGFR agent plus irinotecan-based therapy in the second- or third-line setting showed almost identical median progression-free survival (PFS) inRASwild-type (WT) tumors with tissue or plasma samples analyzed (8.9 months vs 8.7 months).4In the frontline, the use of cetuximab and leucovorin/5-fluorouracil/ irinotecan produced similar results inRAS-WT andRAS-mutant tumors, regardless of the sequencing method used.5Liquid biopsies may be used as a signal to rechallenge patients who develop a resistance mutation. For some, withdrawal of therapy post progression can cause a decline in theRASmutant allele frequency. In the CRICKET study, stopping anti- EGFR therapy in patients withBRAF- andRAS-WT disease (n = 28) and rechallenging with a similar agent produced a 21% overall response rate.6
“Of the 6 responders, none had aRASmutation in their circulation,” Tie said. This translates to a longer PFS in patients who wereRASWT by ctDNA analysis prior to rechallenge, she said. “This could potentially be used as a bio- marker for rechallenging patients [with the same therapy].”
References
- Tie J. Liquid biopsy in colorectal cancer. Presented at: ASCO: Medical Crossfire®: How to Use Liquid Biopsies in Oncology Care, hosted by Physicians’ Education Resource®, LLC; May 31, 2019; Chicago, IL.
- Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer.Sci Transl Med. 2016;8(346):346ra92. doi: 10.1126/scitranslmed.aaf6219.
- Tie J, Cohen J, Wang Y, et al. Serial circulating tumor DNA (ctDNA) analysis as a prognostic marker and a real-time indicator of adjuvant chemotherapy (CT) efficacy in stage III colon cancer (CC).J Clin Oncol. 2018;36(suppl 15; abstr 3516). doi: 10.1200/JCO.2018.36.15_suppl.3516.
- Grasselli J, Elez E, Caratù G, et al. Concordance of blood- and tumor-based detection ofRASmutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017;28(6):1294-1301. doi: 10.1093/annonc/mdx112.
- Normanno N, Esposito Abate R, Lambiase M, et al.RAStesting of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann Oncol. 2018;29(1):112-118. doi: 10.1093/annonc/mdx417.
- Cremolini C, Rossini D, Dell’Aquila E, et al. Rechallenge for patients withRASand BRAFwild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2018;5(3):343-350. doi: 10.1001/jamaoncol.2018.5080.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More