Clinical Findings Identify Associations Between Genomic Correlates and Outcomes in Metastatic CRPC
A study aimed at exploring genomic diversity and defining its associations with clinical outcomes in metastatic castration-resistant prostate cancer found that alterations in RB1 were a potent predictor of poor survival and that other common aberrations may serve as prognostic indicators of treatment response, according to recent work published in the Proceedings of the National Academy of Sciences.
Peter S. Nelson, MD

Peter S. Nelson, MD
A study aimed at exploring genomic diversity and defining its associations with clinical outcomes in metastatic castration-resistant prostate cancer (mCRPC) found that alterations inRB1were a potent predictor of poor survival and that other common aberrations may serve as prognostic indicators of treatment response, according to recent work published in theProceedings of the National Academy of Sciences.1
Study investigators included whole-exome sequencing (WES), gene-expression data, and histopathology to explore how various classes of mCRPC-associated mutations correlated with disease outcomes. Patients were assessed for time on treatment with a next-generation andro- gen receptor signaling inhibitor (ARSI), either enzalutamide (Xtandi) or abiraterone acetate (Zytiga), and their overall survival (OS) time from the date of biopsy.1
Efforts to define the heterogeneity of mCRPC have identified several characteristic mutation profiles associated with the development of the disease, including frequent mutations in the androgen receptor (AR)gene, E26 transformation-specific (ETS) transcription factor genes, and DNA repair genes.
As previously observed, aberrations inAR, ETS transcription factorsERGandETV1, and tumor-suppressor genesTP53,PTEN, andRB1were observed most frequently, along with mutations in genes involved in cell-cycle, epigenetic, PI3K, or DNA repair pathways. The use of WES, however, allowed for expansion of this information to explore mutations that co-occurred frequently in tumors or exhibited mutual exclusivity, illuminating relationships between genes and pathways in the development of the disease.
Peter S. Nelson, MD, Endowed Chair for Prostate Cancer Research at the Fred Hutchinson Cancer Research Center, genitourinary oncology clinical research director at Seattle Cancer Care Alliance, and 1 of the corresponding authors on the paper, was surprised by the power of associations. “This is the largest study of prostate cancer metastases to date,” Nelson said in an interview withTargeted Therapies in Oncology. “The study improved our understanding of the frequency of particular genomic aberrationsfrom rare to common—and emphasized areas for drug development in the future to take advantage of these aberrations.”
The identification of different genomic subtypes of mCRPC and the subsequent response to first-line ARSI treatment are important considerations for therapy. “The study reinforced that prostate cancer, particularly metastatic prostate cancer, is not a single disease but, rather, com- posed of many different subtypes,” Nelson said. “Most subtypes respond to androgen-receptor targeting, though particular molecular features provide an indication of how long a patient is likely to respond.”
Nelson also suggested that the work may serve to inform novel areas of research for improved therapeutic options for subsets of patients who harbor certain mutations and may inform how providers approach treatment to achieve optimal patient outcomes. “[Subtypes] that are likely to respond to specific drugs, such as those withBRCA1/2mutations, are common enough to warrant routine testing,” he said. “Other subtypes do not yet have specific therapeutics, and that is an area of active research to be aware of in the future.”
Providers, therefore, would benefit from an awareness of the genomic landscape of mCRPC and the common aberrations observed, their effects on disease outcome, and strategies for improving outcomes among various patient subtypes.
Tumor Suppressor Genes
Prior data showed enrichment of aberrations in tumor suppressor genes in mCRPC, with compound hits in multiple genes associated with poor patient outcomes.2The present study similarly found a high fraction of oncogenic mutations, particularly in the TP53andPTENgenes. Alterations inTP53primarily consisted of single-nucleotide variations, whereasPTENandRB1more frequently experienced deep deletions.
The authors also found a high co-occurrence of alterations withinTP53andRB1and between mutations inPTENand the ETS family transcription factorERG. A tendency toward mutual exclusivity was observed between mutations inRB1andAR, which were independently associated with a shorter time on ARSI treatment. Alterations inTP53also were associated with a shorter course of ARSI treatment but not with OS.
The study found the strongest association with clinical outcomes with genomic alterations inRB1, which were linked with a shorter time on ARSI treatment and a shorter OS. Concurrent loss ofRB1andTB53was also associated with a higher neuroendocrine expression score and histologic neuroendocrine features, though the concordance was imperfect.
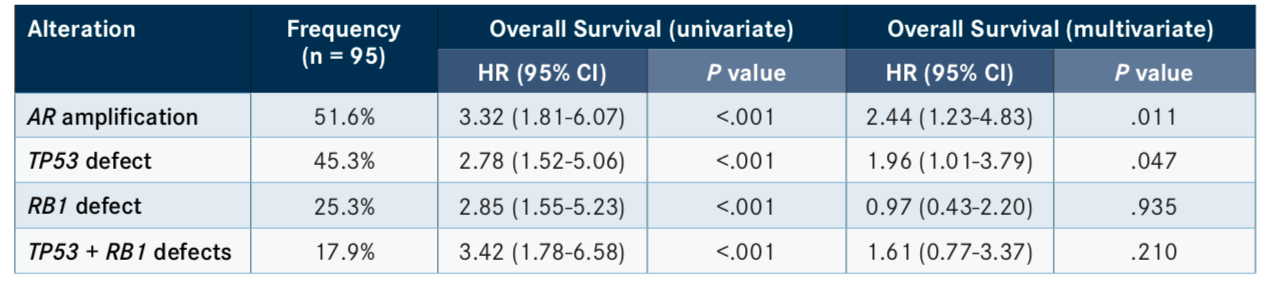
Similarly, other recent studies have seen failure with ARSI treatment in patients harboring mutations in tumor-suppressor genes. According to the results of a phase II clinical trial presented at the 2019 American Society of Clinical Oncology Annual Meeting, in which patients were randomized to receive cabazitaxel (Jevtana) or an ARSI, those with defects inTP53, especially with concurrent mutations inRB1, exhibited poor progression-free survival (PFS) and OS (TABLE).3
Additional data presented at the meeting found that alterations inRB1were strongly predictive of poor response to both ARSI treatment and docetaxel chemotherapy, with compound mutations inPTENandRB1further driving disease progression on ARSI therapy.4The poor response to ARSI treatment in individuals harboring alterations in RB1and other tumor suppressor genes underscores the need for differential treatment options for this patient subset.
Alternatives that do not focus on directly targeting ARs may represent an improved therapeutic option for patients harboring mutations in tumor-suppressor genes, particularlyRB1. Current research in this field has been limited, but exploration of AR-targeting therapies in the treatment of mCRPC has shown poor responses in patients with these mutations.3,4One promising avenue of research for patients withRB1mutations may be the use of hypomethylating agents. In addition to other types of aberrations,RB1is frequently found to be methylated and deleted in CRPC.5The hypomethylating agent 5-azacitidine (Vidaza) was shown to decrease global genomic (LINE-1) DNA methylation in patients with mCRPC in a phase II clinical trial.6Although further testing is needed, exploration of this line of treatment in mCRPC may warrant investigation.
Androgen Receptor
As expected, aberrations inARwere also commonly observed, frequently in the form of amplifications and mutations, which tended toward mutual exclusivity with mutations inRB1. Similar toTP53, mutations inARwere associated with shorter time on ARSI treatment but did not correlate with OS. However, genomic alterations were more frequent in patients previously treated with ARSIs compared with ARSI-naive tumors, suggesting a class of tumors resistant to next-generation ARSIs.
Differential splice variants ofARwere observed, includingARsplice variant 7 (AR- V7), which was previously associated with poor clinical outcomes in response to ARSIs.7However, the current study found no link between expression of AR-V7 and either time on ARSI treatment or OS, indicating further investigation is needed into the clinical utility of this transcript as a diagnostic tool.
Tumors with mutations inARexhibiting resistance to enzalutamide and abiraterone acetate suggest incomplete targeting of overactive ARs by these compounds. The poor prognosis of patients with alterations inARtreated with abiraterone acetate or enzalutamide in a phase II clinical trial supports the idea of a resistant subset ofARaberrations. For these patients, a combination approach with ARSIs added to chemotherapy may be appropriate.3
Several new AR-targeted therapies are in development for use in the treatment of CRPC.8Perhaps the most promising at present is the AR antagonist darolutamide; in a recently completed phase III clinical trial, the agent improved OS and metastasis-free survival in patients with nonmetastatic CRPC.9Based on these data, darolutamide was recently granted priority review by the FDA for this indication.10
Other Gene Families
Cell-Cycle Regulators
Of the genes involved in cell-cycle regulation,CDK12was found to have a high frequency of oncogenic mutations in this cohort. Interestingly, however, loss-of-function mutations inCDK12showed strong evidence of co-occurrence with amplification of 2 other cell- cycle regulatorsCCND1andCDK4. This suggests potential use of CDK4/6 inhibitors in treatment of tumors with mutated CDK12, though further investigation into this relationship is needed.1
ThePIK3CAgene was also found to have high frequency of oncogenic mutations. The PI3K/AKT signaling pathway is frequently found to be dysregulated in prostate cancer; as a result, clinical trials are under way to test the use of the AKT inhibitor AZD5363 in patients with mCRPC and other solid tumors (NCT02525068, NCT03310541). However, mutations in any PI3K pathway genes were not found to be associated with disease outcomes in the current patient cohort and may not be associated with response to treatment with ARSIs.
ETS Family Transcription Factors and SPOPMutations
Prostate cancers with ETS fusions andSPOPmutations represent distinct genomic subtypes of prostate cancer, with differential treatment options and prognoses.11 Prostate cancers with characteristic SPOPmutations were previously shown to be associated with a favorable prognosis; however, a survival benefit was not observed in the current cohort. There was, however, an association with mutations inSPOPand a longer time on ARSI treatment, consistent with observations showing enrichment ofSPOPmutations in earlier disease relative to mCRPC.11These results suggest that SPOPmutations may serve as a diagnostic marker for disease stage but do not inform overall prognosis.
DNA Repair Genes
Unlike previous findings, mutations in DNA repair genes (BRCA1, BRCA2,andATM) were not associated with clinical outcomes on ARSIs, despite high rates of oncogenic mutations, particularly inBRCA2. However, tumors with this class of mutations may respond better to more targeted therapeutic options. Clinical trials are testing the utility of PARP inhibitors, such as olaparib (Lynparza) and niraparib (Zejula), in patients with DNA-repair abnormalities. In the phase II GALAHAD trial (NCT02854436), single-agent niraparib produced an objective response rate of 38% of patients with mCRPC and mutations inBRCA1/2.12In patients unselected for DNA damage alterations previously treated with chemotherapy, pembrolizumab (Keytruda) plus olaparib produced 2 partial responses in 28 patients, leading trial investigators to conclude that the combination has promising activity.13
Nelson emphasized that although under- standing of the genomic landscape of mCRPC has come a long way, there is still much left to learn. “The next step is to link the genomic aberrations and gene expression data with more detailed clinical data, with respect to responses to specific therapeutics, molecular resistance mechanisms, [and] tumor heterogeneity, [and] evaluate the original primary tumors that gave rise to the metastases to better understand what drivers led to the metastatic process.”
References
- Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer.Proc Natl Acad Sci U S A. 2019;116(23):11428-11436. doi: 10.1073/pnas.1902651116.
- Hamid AA, Gray KP, Shaw G, et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer [published online December 12, 2018].Eur Urol. doi: 10.1016/j.eururo.2018.11.045.
- Chi KN, Taavitsainen S, Iqbal N, et al. Updated results from a randomized phase II study of cabazitaxel (CAB) versus abiraterone (ABI) or enzalutamide (ENZ) in poor prognosis metastatic CRPC.J Clin Oncol. 2019;37(suppl 15; abstr 5003). doi: 10.1200/JCO.2019.37.15_suppl.5003.
- Hamid A, Beltran H, Choudhury AD, Sweeney C. Genomic predictors of benefit of docetaxel (D) and next-generation hormonal therapy (NHT) in metastatic castration resistant prostate cancer (mCRPC).J Clin Oncol. 2019;37(suppl 15; abstr 5018). doi: 10.1200/JCO.2019.37.15_suppl.5018.
- Friedlander TW, Roy R, Tomlins SA, et al. Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer Res. 2012;72(3):616-625. doi: 10.1158/0008-5472.CAN-11-2079.
- Sonpavde G, Aparicio AM, Zhan F, et al. Azacitidine favorably modulates PSA kinetics correlating with plasma DNA LINE-1 hypomethylation in men with chemonaïve castration-resistant prostate cancer.Urol Oncol. 2011;29(6):682-689. doi: 10.1016/j.urolonc.2009.09.015.
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer.N Engl J Med. 2014;371(11):1028-1038. doi: 10.1056/NEJMoa1315815.
- Dellis AE, Papatsoris AG. Perspectives on the current and emerging chemical androgen receptor antagonists for the treatment of prostate cancer.Expert Opin Pharmacother. 2019;20(2):163-172. doi: 10.1080/14656566.2018.1548611.
- Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer.N Engl J Med. 2019;380(13):1235-1246. doi: 10.1056/NEJMoa1815671.
- U.S. FDA accepts new drug application and grants priority review for darolutamide. [news release]. Whippany, NJ: Bayer; April 29, 2019. bit.ly/2IMTZFJ. Accessed June 9, 2019.
- Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer.Cell. 2015;163(4):1011-1025. doi: 10.1016/j.cell.2015.10.025.
- Smith MR, Sandhu SK, Kelly WK, et al. Phase II study of niraparib in patients with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD): preliminary result of GALAHAD.J Clin Oncol. 2019;37(suppl 7; abstr 202). doi: 10.1200/JCO.2019.37.7_suppl.202.
- Yu EY, Massard C, Retz M, et al. Keynote-365 cohort A: pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC).J Clin Oncol. 2019;37(suppl 7; abstr 145). doi: 10.1200/JCO.2019.37.7_suppl.145.

Checkpoint Inhibitors Deliver Longer OS in PPP2R1A-Mutated Endometrial Cancer vs Wild-Type Disease
April 7th 2025Dual checkpoint inhibitors led to longer overall survival in patients with high-risk endometrial cancer who displayed inactivating PPP2R1A mutations vs patients with wild-type PPP2R1A.
Read More