VEGFR Targeted Therapies and Combinations Emerge for the Treatment of Sarcomas
Based on the ongoing and positive data observed with VEGFR targeted therapies, novel tyrosine kinase inhibitors and those already approved for other disease indications may become available to patients with bone and soft tissue sarcomas.
Lara E. Davis, MD

Lara E. Davis, MD
Targeted agents with activity against the VEGF pathway are showing promising efficacy in the treatment of bone and soft tissue sarcomas (STS) and may offer new therapy options for patients with these rare tumor types.
Although investigators are working to determine which patients see the most benefit from VEGF inhibition, the data indicate that it has activity in patients across most sarcoma types.
STS is a highly vascular disease, and inhibiting angiogenesis by way of the VEGF signaling pathway and corresponding receptors (VEGFRs) has shown promising activity in sarcomas.1 Prior research revealed that high expression of VEGFR2 is significantly associated with lower rates of patient survival, and platelet-derived growth factor receptor ß and VEGFR1/2/3 are significantly correlated with higher tumor grading.2
The current standard of care in both STS and osteosarcoma includes surgical resection when possible and systemic therapy. In osteosarcoma, the standard of care includes chemotherapy combinations.3
Pazopanib (Votrient), an antiangiogenetic agent, is the only targeted therapy with an FDA-approved indication and activity against VEGFR in nongastrointestinal stromal tumor (GIST) sarcomas. Additionally, the National Comprehensive Cancer Network Guidelines recommend regorafenib (Stivarga) as a single-agent regimen in STS with nonspecific histologies based on the phase II REGOSARC trial, in which the agent significantly extended progression-free survival (PFS) compared with placebo in patients with non-GIST STS stubtypes.4
Based on the ongoing and positive data observed with VEGFR targeted therapies, novel tyrosine kinase inhibitors (TKIs) and those already approved for other disease indications may become available to patients with bone and soft tissue sarcomas.
Preoperative Pazopanib in STS
Investigators on a phase II study in treatment-naïve patients with resectable, nonmetastatic, high-risk STS tested whether preoperative devascularization would improve local control and outcomes following surgery. Pazopanib, a TKI with antiangiogenic properties, was administered preoperatively in 21 patients, and although the study did not show effectiveness in the overall cohort, relevant treatment effects were seen in a single patient, suggesting that efficacy in this setting may apply to certain patient subgroups.5
Results from the phase II ARST1321 study, which used chemoradiation with or without pazopanib preoperatively in adult and pediatric patients ≥2 years with unresected and newly diagnosed nonrhabdomyosarcoma STS, were presented at the 2019 American Society of Clinical Oncology Annual Meeting. Patients received preoperative radiation therapy and ifosfamide plus doxorubicin, with half of patients receiving concurrent pazopanib, followed by resection at week 13 and then adjuvant chemotherapy. The rate of near complete pathologic response (≥90%) was significantly greater at 58.3% with the addition of pazopanib versus 22.2% with chemoradiation only (P= .02).6
“There seems to be a role in making these tumors potentially more susceptible to chemotherapy and probably radiotherapy as VEGF inhibitors modify the tumor vasculature,” said Lara E. Davis, MD, assistant professor of medicine at Oregon Health & Science University in Portland, in an interview withTargeted Therapies in Oncology.
Breelyn A. Wilky, MD

Breelyn A. Wilky, MD
Regorafenib in Bone Sarcomas
Regorafenib inhibits the activity of VEGFR1/2/3 and other kinases in osteosarcoma. In the osteosarcoma cohort of the phase II SARC024 study led by Davis, 42 adult patients with measurable disease by RECIST who had ≥1 prior therapy were randomized to either placebo or regorafenib. Results showed that median PFS with regorafenib was 3.6 months compared with 1.7 months with placebo (HR, 0.42; 95% CI, 0.21-0.85;P= .017).7The investigators concluded that regorafenib should be considered a treatment option in this population. “In regorafenib, the doubling of PFS... indicates that this tumor has some dependency on these pathways,” Davis said. In the same study, regorafenib is being tested in patients with Ewing sarcomas, liposarcomas, and rhabdomyosarcomas; positive results have already been reported in the Ewing-sarcoma arm and the trial is ongoing.8
This regimen did not appear to be much more toxic than existing chemoradiation regimens. “I will be interested to see how the toxicity plays out in older adults in that population,” Davis saidbalancing the toxicity with efficacy is important.
The phase II REGOBONE study examined the safety and efficacy of regorafenib for progressive metastatic disease after ≥1 prior treatment in 4 independent arms: osteosarcoma, Ewing sarcoma, chondrosarcoma, and chordoma. In the osteosarcoma cohort, the primary outcome measure was the proportion of patients showing no disease progression at 8 weeks, with 65% in the regorafenib arm (n = 26) and none in the placebo group (n = 12) reaching that endpoint. The other trial arms are ongoing.9
Median PFS was 16.4 weeks (95% CI, 8.0-27.3) for those in the regorafenib arm compared with 4.1 weeks (95% CI, 3.0-5.7) in the placebo arm. Median OS was 11.3 months (95% CI, 5.9-23.9) with regorafenib versus 5.9 months (95% CI, 1.3-16.4) with the placebo. Investigators suggested that regorafenib has activity in advanced disease and in those with a high risk of relapse and is a potential complementary agent with chemotherapy.9
“We have known for a while that multikinase inhibitors seem to have an impact in the relapsed and refractory setting for osteosarcoma,” said Davis, adding that osteosarcoma is a genomically complex tumor. “Certainly, there is not only 1 growth factor responsible, but changing the microenvironment and some growth signals is seeming to help patients, with a reasonable toxicity.” Even if multiple pathways play a role in the tumorigenesis of osteosarcoma, “one constant is VEGF,” she said.
Davis noted a small 2011 study of 35 patients with previously treated, high-grade osteosarcoma in which sorafenib (Nexavar) produced a median PFS of 4 months and a median OS of 7 months.10A 2015 study of 38 patients showed that sorafenib with everolimus (Afinitor) for patients with previously treated osteosarcoma produced significant toxicities, and the trial did not meet the prespecified target of 6-month PFS of ≥50%. However, patients with tumors that overexpressed posphorylated (P) ERK1/2 and P–RPS6 had the greatest benefit from therapy, leading the investigators to be optimistic about advancements in the knowledge of osteosarcoma biology for targeting specific pathways.11
Davis said that in osteosarcoma, investigators have had a difficult time getting immunotherapy to work, but small signals were observed in past studies.12“Investigators are interested in how these 2 methods [immune and targeted therapy] can be combined in a rational and synergistic way to improve osteosarcoma outcomes,” she said. Starting in the metastatic setting, with TKIs like regorafenib combined with immunotherapy, it’s possible that these regimens will eventually be moved to the frontline setting.
Apatinib for STS and Osteosarcoma
A recent phase II trial of the novel VEGFR2 inhibitor apatinib (YN968D1) in metastatic sarcoma focused on safety and efficacy in 64 patients who had previously failed on chemotherapy. The median PFS was 7.93 months with a PFS rate of 74% at 12 weeks. The final objective response rate (ORR) was 15.25% with a disease-control rate of 57.63% in 59 evaluable patients, leading trial investigators to conclude that the TKI was effective and well tolerated. Although no patients showed a complete response (CR), 25.49% had a partial response (PR) and 70.59% showed stable disease at some point.13
Prior to this study, a review of clinical and preclinical data for apatinib showed that current evidence points to the agent’s potential superiority over other targeted antiangiogenesis drugs for sarcoma, including pazopanib, sorafenib, and sunitinib (Sutent).14
One recent phase II study showed the combination of apatinib plus camrelizumab, an antiPD-1 immune checkpoint inhibitor, extended PFS in patients with osteosarcoma over apatinib alone. The results in 41 patients showed an ORR of 21.95%.15
“We are hopeful it is a signal for something exciting,” Davis said. “We should learn more from figuring out how apatinib compares to other TKIs in targets and efficacy.”
Axitinib/Pembrolizumab Combination in Advanced Sarcomas
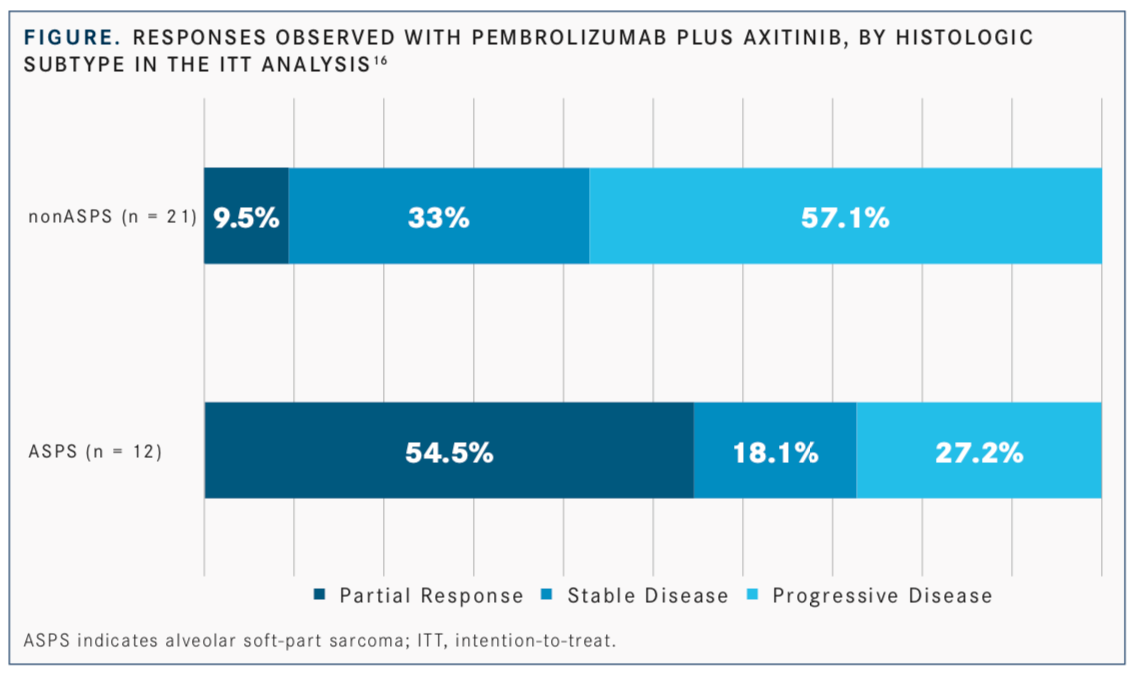
In a phase II single-arm trial assessing the TKI axitinib (Inlyta) plus the antiPD-1 immune checkpoint inhibitor pembrolizumab (Keytruda) in 33 patients with advanced or metastatic sarcomas, including alveolar soft-part sarcoma (ASPS), the combination showed promising efficacy and a manageable toxicity profile. Patients had previously been treated with ≥1 prior systemic therapy, except for those who declined therapy or for whom no standard treatment existed. With a median follow-up time of 14.7 months, the primary endpoint of 3-month PFS was achieved in 65.5% of patients in the overall cohort, 72.7% of patients with ASPS, and 61.9% of patients with non-ASPS disease. In the total cohort, 25% of patients achieved an objective response, made up of all partial responses (FIGURE).16
“Less than 20% of sarcomas will respond to these immune checkpoint drugs by themselves,” said the trial’s primary investigator, Breelyn A. Wilky, MD, associate professor of medicine/medical oncology at University of Colorado School of Medicine in Aurora. “We think that may be because there is a lot of VEGF in sarcomas, which may create a negative environment to get immune cells where they need to go.”
Investigators have been combining immunotherapy and VEGF inhibitors in multiple cancer types, especially renal cancer, she said. Published studies have shown that patients with kidney cancer have better outcomes with this particular drug combination versus either of the drugs alone.17“That is what we wanted to test with sarcomas,” she said.
When asked why patients with ASPS had the best responses, Wilky said, “ASPS is a simpler sarcoma, with only 1 genetic abnormalitytranslocation. When those 2 genes come together, they start increasing other proteins in the cell, and several are blood vesseltype proteins like VEGF. We think in other types of sarcomas, there is a lot else going on; they are not as dependent on VEGF as ASPS.”
In Wilky’s study, 25% of all patients (54.5% with ASPS; 9.5% with non-ASPS) treated with the combination had a PR15; this was the first trial for this drug combination for ASPS or sarcomas in general, and some of the patients had responses lasting more than a year. One patient still has no detectable disease after being off treatment for more than a year, though previously she had rapidly growing disease.
Combination Therapy in GISTs
The single-arm, phase II CaboGIST trial in 50 patients with GISTs who progressed on imatinib (Gleevec) and sunitnib (Sutent) and received cabozantinib (Cabometyx) met its primary endpoint with a 58.5% progression-free rate at 12 weeks. A median PFS and OS of 6.0 months (95% CI, 3.6-7.7) and 14.4 months (95% CI, 14.1-not evaluable), respectively, were observed. Although no CRs were achieved, 14% of patients had a PR and 66% had stable disease, for a clinical-benefit rate of 80%.18
“The study showed nice results in patients with GIST. This is the first time the drug has been shown effective in these patients,” Wilky said. She added that she looks forward to seeing confirmation of the results in other studies.
Treatment Outlook
Determining which sarcoma types or patients will most benefit from VEGFR inhibition is challenging, but this has not dampened the successes observed in many patients. “When it works well, patients can be stable on the drugs for years,” Wilky said.
Multiple antiangiogenic agents have clinical activity in repressing sarcoma growth, though none of the drugs tested or used so far have emerged as a home run, she said. It is difficult to know how to prioritize which drug to use for each patient or sarcoma type, she said: “We don’t really know how to tease apart which ones are useful.” That is partly because no one knows what proteins are affected by the treatment, and so far, no studies prove that VEGF is the most important factor to target when treating sarcomas. “Most of these drugs hit a dozen different proteins,” Wilky said. “Overall, we know that VEGF [expression] is a bad prognostic sign. Anything that suppresses blood vessel growth will be helpful.”
Studies have shown that VEGF inhibition is most effective in vascular sarcomas, Davis said. Those with advanced angiosarcomas may have success with pazopanib or bevacizumab (Avastin), as have those with solitary fibrous tumors, which respond to VEGF inhibition in advanced settings.
In the future, investigators may need to try adding more drugs to their combination regimens, such as 2 immune checkpoint inhibitors working at different phases in the process, Davis said. The treatment outlook in these tumors may involve chemotherapy, targeted therapies, and then checkpoint inhibitors.
Combinations with chemotherapy are also interesting, Wilky said. One problem with using immunotherapy is that the immune cells may not easily recognize sarcoma cells because they look like normal cells. The signals and inflammation created by chemotherapy allow the immune system to better recognize the cancerous cells.
Wilky also thought that future trials will focus on subsets of sarcomas, since sarcomas are varied. “It is like studying 100 different diseases,” she said, and it is challenging to include multiple sarcoma types in a small study. Instead, smaller studies can look at 1 or 2 subtypes.
References
- Nguyen DT, Shayahi S. Pazopanib: approval for soft-tissue sarcoma. J Adv Pract Oncol. 2013;4(1):53-57. bit.ly/2xfsI7c.
- Kampmann E, Altendorf-Hofmann A, Gibis S, et al. VEGFR2 predicts decreased patients survival in soft tissue sarcoma.Pathol Res Pract. 2015;211(10):726-730. doi: 10.1016/j.prp.2015.04.015.
- Chemotherapy for osteosarcoma. American Cancer Society website. bit. ly/2WFD5Ab. Updated January 31, 2018. Accessed June 11, 2019.
- NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma, version 2.2019. National Comprehensive Cancer Network website. NCCN website. bit.ly/2sxwHJ5. Published February 4, 2019. Accessed June 11, 2019.
- Ronellenfitsch U, Karampinis I, Dimitrakopoulou-Strauss A, et al. Preoperative pazopanib in high-risk soft tissue sarcoma: phase II window-of-opportunity study of the German Interdisciplinary Sarcoma Group (NOPASS/GISG-04).Ann Surg Oncol. 2019;26(5):1332-1339. doi: 10.1245/s10434-019-07183-4.
- Weiss AR, Chi Y-Y, Chen Y-L, et al. Preoperative chemoradiation positive/negative pazopanib in non-rhabdomyosarcoma soft tissue sarcoma (NRSTS): a report from Children’s Oncology Group (COG) and NRG Oncology.J Clin Oncol.2019;37(suppl 15; abstr 11002). doi: 10.1200/JCO.2019.37. 15_suppl.11002.
- Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma.J Clin Oncol.2019;37(16):1424-1431. doi: 10.1200/JCO.18.02374.
- Attia S, Bolejack V, Ganjoo KN, et al. A phase II study of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS)of soft tissue and bone: SARC024 trial results.J Clin Oncol. 2017;35(suppl 15;abstr 11005). doi: 10.1200/JCO.2017.35.15_ suppl.11005.
- Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non- comparative, randomised, double-blind, placebo-controlled, phase 2 study.Lancet Oncol.2018;20(1):120-133. doi: 10.1016/S1470-2045(18)30742-3.
- Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study.Ann Oncol.2012;23(2):508-516. doi: 10.1093/annonc/mdr151.
- Grignani G, Palmerini E, Ferraresi V, et al; Italian Sarcoma Group. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial.Lancet Oncol.2015;16(1):98-107. doi: 10.1016/S1470- 2045(14)71136-2.
- Meyers PA, Chou AJ. Muramyl tripeptide-phosphatidyl ethanolamine encapsulated in liposomes (L-MTP-PE) in the treatment of osteosarcoma.Adv Exp Med Biol.2014;804:307-321. doi: 10.1007/978-3-319-04843-7_17. 13. Liao Z, Li F, Zhang C, et al. Phase II trial of VEGFR inhibitor apatinib for metastatic sarcoma: focus on efficacy and safety.Exp Mol Med.2019;51(3):24. doi: 10.1038/s12276-019-0221-7.
- Liao Z, Li F, Zhang C, et al. Phase II trial of VEGFR inhibitor apatinib for metastatic sarcoma: focus on efficacy and safety.Exp Mol Med. 2019;51(3):24. doi: 10.1038/s12276-019-0221-7.
- Li F, Liao Z, Zhang C, et al. Apatinib as targeted therapy for sarcoma. Oncotarget. 2018;9(36):24548-24560. doi: 10.18632/oncotarget.24647.
- Xie L, Guo W, Xu J, et al. Apatinib plus camrelizumab (SHR-1210) for unresectable high-grade osteosarcoma (APFAO) progressing after chemo- therapy: a prospective, open label, phase II trial.J Clin Oncol. 2019;37(suppl 15; abstr 11013). doi: 10.1200/JCO.2019.37.15_suppl.11013.
- Wilky BA, Trucco MM, Subhawong TK, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-center, single-arm, phase 2 trial.Lancet Oncol. 2019;20(6):837-848. doi: 10.1016/S1470-2045(19)30153-6.
- Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma.N Engl J Med. 2019;380(12):1116-1127. doi: 10.1056/ NEJMoa1816714.
- Schoffski P, Mir O, Kasper O, et al. Activity and safety of cabozantinib in patients with gastrointestinal stromal tumor after failure of imatinib and sunitinib: EORTC phase II trial 1317 CaboGIST.J Clin Oncol. 2019;37(suppl 15;abstr 11006). doi: 10.1200/JCO.2019.37.15_suppl.11006.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More