With Biomarker Expansion, Combinations and Personalized Medicine Continue to Rise
Biomarker expansion has enjoyed a boom since 2006, with patient incidence of positive biomarkers reaching up to 50% in non–small cell lung cancer and melanoma and 25% in acute myeloid leukemia and myelodysplastic syndromes, according to the <em>Global Oncology Trends 2018</em> report.
Michael Kleinrock

Michael Kleinrock
Even as targeted therapies continue to meet regulatory approvals, more and more biomarkers are being used to define cancer more precisely across tumor types. Biomarker expansion has enjoyed a boom since 2006, with patient incidence of positive biomarkers reaching up to 50% in nonsmall cell lung cancer (NSCLC) and melanoma and 25% in acute myeloid leukemia and myelodysplastic syndromes, according to theGlobal Oncology Trends 2018report (FIGURE 1).1Since 2006, investigators have identified additional biomarkers in non-small cell lung cancer, colorectal cancer, gastric cancer, melanoma, acute myeloid leukemia, myelodysplastic syndrome, chronic lymphocytic leukemia, breast cancer, and prostate cancer.
Previously, many tumors were treated with general treatments such as surgery, radiation, or chemotherapy, but now testing for biomarkers is helping oncologists with more personalized treatment selections. Notably, prior to 2006, biomarker expansion was highlighted with patient incidences of biomarkers such as Philadelphia chromosome positivity, seen in up to 95% of patients with chronic myeloid leukemia; HER2-negative, HR-positive breast cancer found in up to 53% of postmenopausal patients; and wild-typeKRASfound in up to 50% of patients with colorectal cancer.
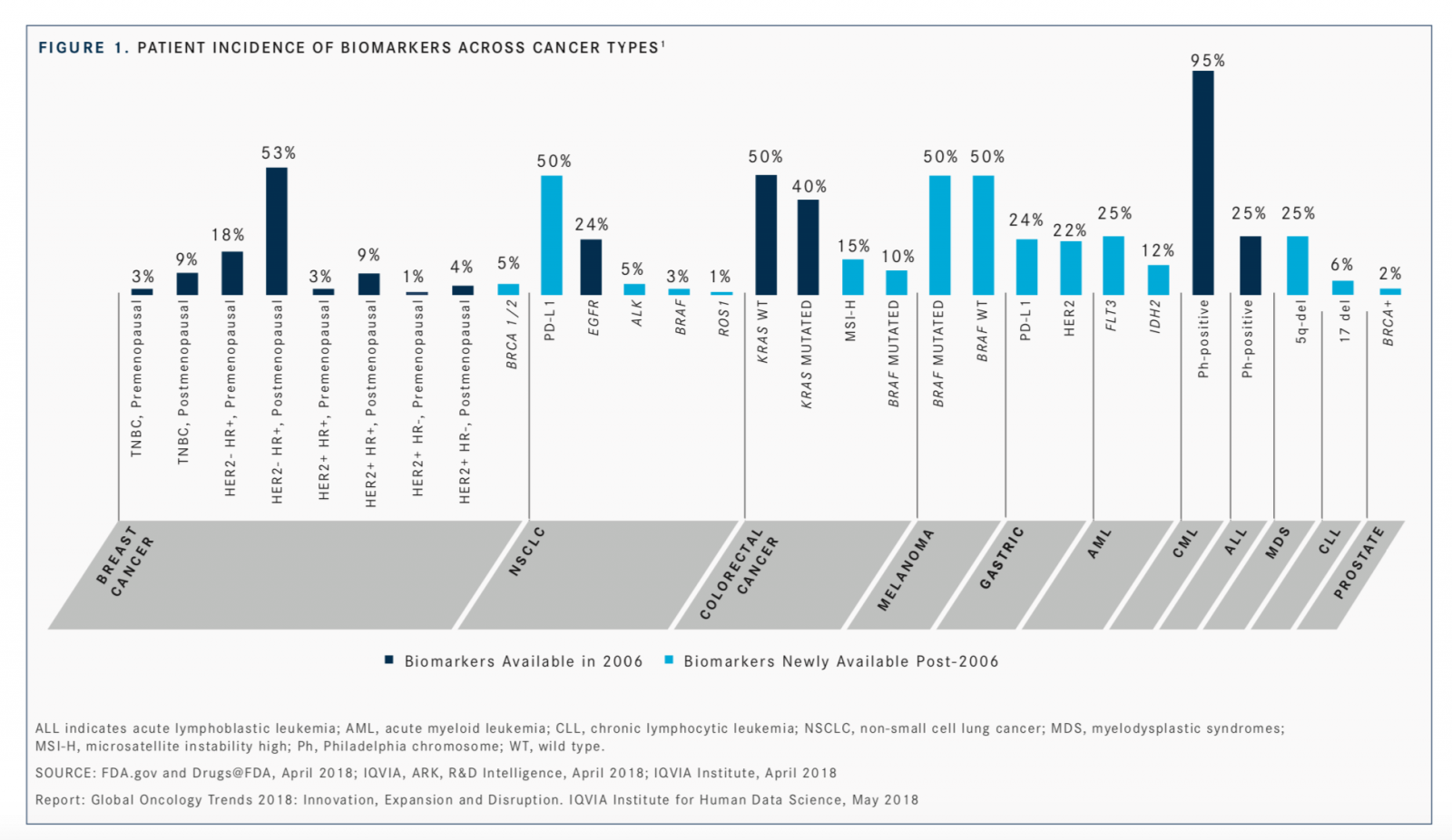
“Breast cancer was highly segmented even in the early 2000s, but the addition of theBRCA1/2genetic marker further isolates responders to specific treatments. The addition of PD-L1 expression [as a biomarker] across a range of tumors has enabled identification of responsive patient subpopulations, with more demonstrable clinical effects,” said Michael Kleinrock, director of research development at the IQVIA Institute for Human Data Science, said during a presentation of the report.
With the expansion of agent approvals and greater biomarker data, the report notes a rise in personalized medicine, specifically the use of biomarkers to further stratify patients.
“What we’re also seeing in terms of innovation in some of the more common tumors is more precise targeting for specific medicines, with all the different biomarker tests or assays available now that [were] not available 10 years ago,” Kleinrock said.
Since 2017, the FDA has approved the use of 55 newly created hematology and oncology agents to treat 68 types of cancer, with some drugs having multiple indications, eg, PD-1 and PD-L1 checkpoint inhibitors, which have broad efficacy across solid tumors and multiple indications across tumor types.2,3The addition of PD-L1 expression across a range of tumors has allowed oncologists to identify those patient subpopulations that could be more responsive to treatment and result in optimal outcomes.
Of the 14 new cancer agents launched in 2017, all were targeted therapies and 11 were granted breakthrough therapy designations by the agency.1As a whole, the group showed an improved remission rate in some cancer types above 50%, with incremental survival demonstrated in those tumor types in which rates were already high.
Of significance is the increased accessibility of checkpoint inhibitors demonstrating a remarkable clinical profile and continued expansion in combination therapy in NSCLC, colorectal, gastrointestinal, liver, and urothelial carcinoma.
The flurry of regulatory approval activity, including new indications for previously approved agents and the use of unique combinations with these agents, has contributed to their expansion.
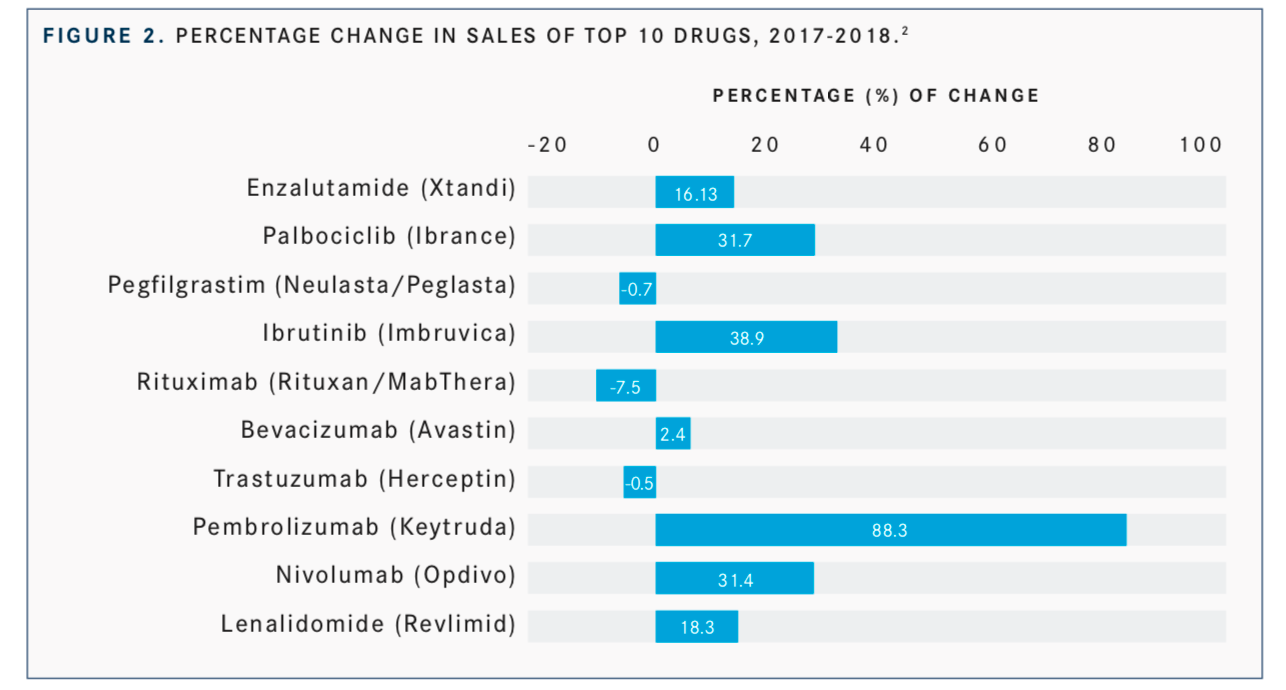
For example, the multiple indications for the immuno-oncology drugs, namely pembrolizumab (Keytruda) and nivolumab (Opdivo) in NSCLC, melanoma, and renal cell carcinoma, has resulted in an increase in their sales from 2017 to 2018. Specifically, pembrolizumab enjoyed the biggest increase in sales going from $3.81 billion in 2017 to $7.17 billion in 2018, for an increase of 88.3% and nivolumab increased sales from $5.76 billion to $7.17, resulting in an increase of 31.4% among a list of the top 10-cancer selling drugs (FIGURE 2).2
Kleinrock’s outlook is optimistic. “Patients are able to use greater specific and likely... more successful treatment options, so [these are] exciting times for those patients if the new medicines actually work. Outcomes are improving quite significantly,” he said.
References
- IQVIA Institute for Human Data Science. Global oncology trends 2018: innovation, expansion and disruption. iqvia.com/institute/reports/glob- al-oncology-trends-2018?_ga=2.187228842.32077673.1556213549- 263456235.1556039863. Published May 24, 2018. Accessed May 1, 2019. 2. Genetic Engineering & Biotechnology News. https://www.genengnews. com/a-lists/top-10-best-selling-cancer-drugs-of-2018/.
- 2019 FDA Approved drugs. CenterWatch website. centerwatch.com/drug-information/fda-approved-drugs/year/2019. Accessed May 1, 2019.