Levy Discusses Treatment Options for ALK-Rearranged NSCLC
During a Targeted Oncology case-based roundtable event, Benjamin Levy, MD, discussed the current targeted treatment options that target lung cancers that are positive for ALK rearrangements.
Benjamin Philip Levy, MD
Associate Professor of Oncology
Clinical Director of Medical Oncology
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
Sibley Memorial Hospital
Baltimore, MD

CASE
- A 55-year-old woman presented with worsening dyspnea and persistent dry cough.
- Smoking history: prior smoker (< 5 pack years)
- Medical history: atrial fibrillation
- Imaging revealed a lung mass with metastases to intrathoracic lymph nodes and liver.
- Brain MRI: several subcentimeter intracranial lesions consistent with metastatic disease; she was asymptomatic from her brain metastases.
- Liver biopsy: consistent with lung adenocarcinoma
- Tumor next-generation sequencing (NGS; FoundationOne): ALK (EML4-ALK translocation) positive; negative for EGFR, ROS1, BRAF, KRAS, NTRK, MET, RET
- PD-L1: 0%
Targeted OncologyTM: What do the National Comprehensive Cancer Network guidelines recommend for ALK-rearranged non–small cell lung cancer (NSCLC)?
LEVY: Alectinib [Alecensa], brigatinib [Alunbrig], and lorlatinib [Lorbrena] are all category 1 for first-line indication.1 There are other recommendations—ceritinib [Zykadia] is a category 1. “Useful in certain circumstances” [is the designation] for crizotinib [Xalkori]. But I think the ones that we wrestle with, and the ones that we think about, are alectinib, brigatinib, and erlotinib.
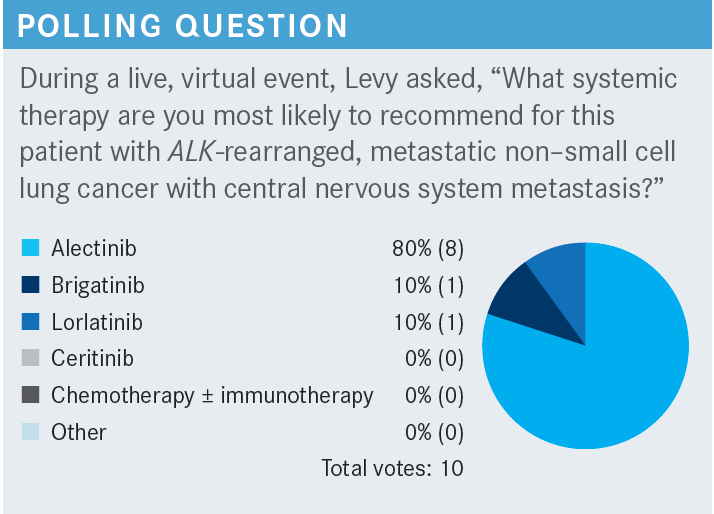
What are the data behind alectinib for this patient population?
The ALEX trial [NCT02075840] was an ALK fusion–positive patient population.2 Interestingly, immunohistochemistry [IHC] was done. IHC is a wonderful test for ALK, by the way. It has a high concordance rate with NGS. So for a rapid readout, we do an IHC for ALK sometimes if we suspect it because you can get your answer within a day. These patients were randomly assigned to alectinib 600 mg twice a day or crizotinib 250 mg twice a day. The primary end point was progression-free survival [PFS].
The PFS for alectinib…is 3 years.3 That’s the median time that they’re on drug before they progress. The median overall survival [OS] hasn’t even been reached yet, with 48 months of follow-up. The 5-year OS rate is 62.5% [HR, 0.67; 95% CI, 0.46-0.98; P = .0376].3 That’s incredible. I know this is a rare patient population, but when I started in lung cancer, the median OS for all patients was 12 months. This is what gets things moving and moves the field forward. The PFS rates [for patients with] baseline central nervous system [CNS] metastases were 40% at 3 years with alectinib vs 2.1% with crizotinib.3
For those of you who treat breast cancer and some other tumors, it’s certainly not as good as that, but we’re making a lot of headway. If you look at the OS by subgroup, everyone seemed to benefit. This is a univariate analysis that we see in any study, where it breaks down essentially different patient populations to see whether one particular patient population had a more pronounced benefit of the drug, the experimental arm, alectinib vs crizotinib. Everyone seemed to benefit.
Can you discuss the ALTA-1 trial (NCT02737501) of brigatinib?
This was another study that was recently published, about 2 or 3 years ago, similar to the ALEX study with a similar comparator arm.2,4 Brigatinib, started at 90 mg and then escalated to 180 mg, was compared with crizotinib. We saw an improvement in PFS; the HR was 0.48 [95% CI, 0.35-0.66; P < .0001].5 I think the Kaplan-Meier curves are very similar to [those of] the ALEX trial.
If you look at the brain metastases at baseline, that group seemed to derive a significant benefit with brigatinib vs crizotinib.5 I’m not a big brigatinib user, but I understand the rationale, based on these ALTA-1 data. If we look at intracranial PFS for those patients with brain metastases, these are small numbers of patients, but the HR is 0.29 [95% CI, 0.17-0.51; P < .0001].5 For those who got brigatinib vs crizotinib, median PFS was 2 years [vs 5.6 months, respectfully]. So essentially, brain metastases were under control, if those receiving brigatinib had them at baseline, for close to 2 years before there was progression in the brain.
The objective response rate (ORR) to brigatinib was 74% vs only 62% with crizotinib [OR, 1.73; 95% CI, 1.04-2.88; P = .0342].6 I think the challenge is we don’t have long-term follow-up with ALTA-1 the way we do with ALEX.
The ORR in the brain with brigatinib vs crizotinib was 78% with brigatinib and only 26% with crizotinib [OR, 11.67; 95% CI, 2.15-63.27; P = .00014], it’s remarkable to see those data.6 We don’t have OS maturity the way we do with ALEX, so we’re all waiting to see how these data pan out.
How did patients respond in the clinical trial of lorlatinib?
The CROWN trial [NCT03052608] is the third chapter of next-generation ALK tyrosine kinase inhibitors [TKIs] vs crizotinib.7 We at least have 3 trials that have the same comparator arm of crizotinib, so we can do what we’re never supposed to do, which is cross-trial comparisons, to see which drug may be better. Phase 3 CROWN data were in [patients with] stage IV, ALK-positive disease. They were allowed to have CNS metastases at baseline. They were randomized to lorlatinib, 100 mg a day, vs crizotinib. The primary end point was PFS.
CROWN had the most pronounced benefit we saw compared with brigatinib and alectinib. If you compare the HRs and you see they diverge more dramatically than in the alectinib data and brigatinib data. The PFS HR was 0.27 [95% CI, 0.18-0.39].7,8 These are cross-trial comparisons, so it’s tough to know whether lorlatinib is better than alectinib or brigatinib, but this is the most pronounced PFS we’ve seen in any study that compares a next-generation ALK TKI to crizotinib.
The ORR with lorlatinib was 77%, and the median duration of response has not been reached.8
There were 3 complete responses.7 The lorlatinib intracranial ORR was 66% vs 20% with crizotinib. These are a small number of patients who were enrolled, and this is only a subset of the patients who were enrolled in the entire study.
For those patients who didn’t have brain metastases, who received lorlatinib, 99% of them did not have brain metastases at 3 years, which is better than any data we have with brigatinib or alectinib so far.8 If you look at the patients with brain metastases who received lorlatinib, about 75% of them at 3 years still had control in the brain. The OS curves overlap, but we’ll see what happens here over time.7 It’s very early, but we’ll have to see how it all shakes out.
Can you summarize ALEX, ALTA-1, and CROWN?
Looking at median PFS between these 3 studies, ORR, intracranial response rate, it looks like interestingly enough that CROWN and ALEX have a little bit of an edge over ALTA-1.3,5,8 But the median PFS for baseline brain metastases has not been reached in CROWN, and I think that curve is the one that everyone talks about. You’re able to protect the brain if you receive lorlatinib.
For the adverse events [AEs] between alectinib, brigatinib, and lorlatinib—every drug has different toxicities—with alectinib, we see a lot of liver function test [LFT] abnormalities and peripheral edema.2 For brigatinib, we see some creatine phosphokinase elevations, and LFT abnormalities.3,4 Then lorlatinib has a different set of AEs that are worth noting. Certainly, the cognitive effects in the hyperlipidemia are real, as somebody who has used this drug before.7,8
CASE UPDATE
- The patient received lorlatinib; radiation therapy was initially deferred.
- Four weeks after initiation of lorlatinib: Repeat MRI showed resolution of brain metastases, and patient had a systemic response.
- Patient reported feeling confused and having difficulty with memory/multitasking for the past 10 days.
How do you manage lorlatinib-emergent CNS AEs?
You need to monitor at baseline and schedule a follow-up visit after the first 6 weeks.9-11 [For management], you need to do an MRI, refer to a psychiatrist or therapist if they develop CNS metastasis, review concomitant medications, and consider holding for grade 1 events [and resume at the same or lower dose after recovery]. But for grade 2 and 3, maybe resume at a reduced dose upon recovery. I usually hold and resume at a lower dose.
The first dose decrement is 75 mg, second is 50 mg, and then permanently discontinue. I’ve had a couple of patients who have had major cognitive deficits from this drug. We were able to get them through, and I think [it’s important to be] engaging the family members and warning [them] that this may happen, because the patient can be completely unaware that this is happening. So we usually get patients through, and some patients do fantastic on the drug without it. We don’t know why some patients develop cognitive deficits and why others don’t. The other thing is hyperlipidemia with lorlatinib can be [an issue]. I always start a patient on a statin when I start lorlatinib.
CASE UPDATE
- Lorlatinib was held for 2 weeks and resumed at 75 mg daily after patient reported complete resolution of symptoms.
REFERENCES
1. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497-530. doi:10.6004/jnccn.2022.0025
2. Peters S, Camidge DR, Shaw AT, et al; ALEX Trial Investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829-838. doi:10.1056/NEJMoa1704795
3. Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056-1064. doi:10.1016/j. annonc.2020.04.478
4. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027-2039. doi:10.1056/NEJMoa1810171
5. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16(12):2091-2108. doi:10.1016/j.jtho.2021.07.035
6. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L Trial. J Clin Oncol. 2020;38(31):3592-3603. doi:10.1200/JCO.20.00505
7. Shaw AT, Bauer TM, de Marinis F, et al: CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018-2029. doi:10.1056/NEJMoa2027187
8. Solomon B, Bauer T, Mok T, et al. Updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib vs crizotinib in advanced anaplastic lymphoma kinase (ALK)- positive non-small cell lung cancer (NSCLC). Cancer Res. 2022;82(suppl 12):CT223. doi:10.1158/1538-7445.AM2022-CT223
9. Reed M, Rosales AS, Chioda MD, Parker L, Devgan G, Kettle J. Consensus recommendations for management and counseling of adverse events associated with lorlatinib: a guide for healthcare practitioners. Adv Ther. 2020;37(6):3019-3030. doi:10.1007/ s12325-020-01365-3
10. Lorbrena. Prescribing information. Pfizer; 2021. Accessed October 14, 2022. https:// bit.ly/3gMRmG7
11. Solomon B, Bauer TM, De Marinis F, et al. LBA2 lorlatinib vs crizotinib in the first-line treatment of patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC): results of the phase III CROWN study. Ann Oncol. 2020;31(suppl 4):S1180-S1181. doi:10.1016/j.annonc.2020.08.2282
