Phillips Evaluates Second-Line Therapy Options for R/R DLBCL
During a Targeted Oncology case-based roundtable event, Tycel Phillips, MD, discussed available therapies for a patient with relapsed/refractory diffuse large B-cell lymphoma including CAR T-cell therapy and combination systemic therapies.
Tycel Phillips, MD
Associate Clinical Professor
Division of Lymphoma
Department of Hematology & Hematopoietic Cell Transplantation
City of Hope
Duarte, CA

Targeted OncologyTM: What do the National Comprehensive Cancer Network (NCCN) guidelines recommend for the treatment of relapsed or refractory B-cell lymphomas?
PHILLIPS: The most recently updated NCCN guidelines recommend that patients who relapse less than 12 months after chemotherapy go down a different pathway than our traditional course of salvage chemotherapy and [autologous stem cell transplant; ASCT]. The recommendation is to consider these patients for CAR [chimeric antigen receptor] T-cell therapy if the patients are eligible, able, and willing to go to a CAR T-cell therapy center.1
We have 2 CAR T-cell products in this space that will be options for patients with primary refractory DLBCL [diffuse large B-cell lymphoma] or early relapse DLBCL. We have axicabtagene ciloleucel [Yescarta], which has approval,2 and we have lisocabtagene maraleucel [liso-cel; Breyanzi], which recently gained approval for second-line therapy.3 For patients who aren’t candidates for CAR T-cell therapy, there are other regimens we can consider.1
What studies have assessed the benefit of CAR T-cell therapy in primary refractory DLBCL?
Several recent studies were designed to capture what we consider to be early relapse in primary refractory DLBCL, and they assessed the event-free survival benefit of CAR T-cell therapy vs the standard of care [SOC], which was salvage chemotherapy and ASCT. These studies were ZUMA-7 [NCT03391466], BELINDA [NCT03570892], and TRANSFORM [NCT03575351].
The median ages for all 3 studies were similar, [ranging from 58 to 60 years]. There were different patient populations in all 3 studies, which is something to [consider].4-6 ZUMA-7, which has the most mature data of the 3, did not allow any bridging chemotherapy, so these patients had to have disease stability sufficient to permit apheresis, manufacturing, and receipt of the study drug. There is always going to be some question about the patients enrolled on that study and how they compare [with] what we see in the real-world setting. But the rate of CR [complete response] and progression-free survival [PFS] strongly favored CAR T-cell therapy vs the SOC therapy in this patient population.4
The BELINDA trial of tisagenlecleucel [tisa-cel; Kymriah] vs SOC did allow bridging therapy. However, there was no benefit to tisa-cel in this patient population. [It is worth noting that] tisa-cel is a bit more hampered than the other 2 CAR T-cell products [regarding] delays of manufacturing and delivering this product to patients. I don’t think anybody can say with certainty how that may have contributed to the outcomes in this study, but of the 3 studies, this is the only one that had a negative outcome. It showed no substantial benefit to CAR T-cell therapy.5
Compared with ZUMA-7, the TRANSFORM trial [of liso-cel vs SOC] was smaller [and the data are less mature], but this study did show significant benefits to overall response rate [ORR], CR rate, and PFS for CAR T-cell therapy. The results were recently published in Lancet and led to [the] approval of this agent in the second-line setting.3,6,7
[As a result of] these studies, we have 2 agents that have been approved for second-line treatment of primary refractory or early relapse DLBCL.2,3 We’ll have to consider how this will change our clinical practice, because CAR T-cell therapy is still limited by access for most patients, and there’s still a delay with manufacturing.

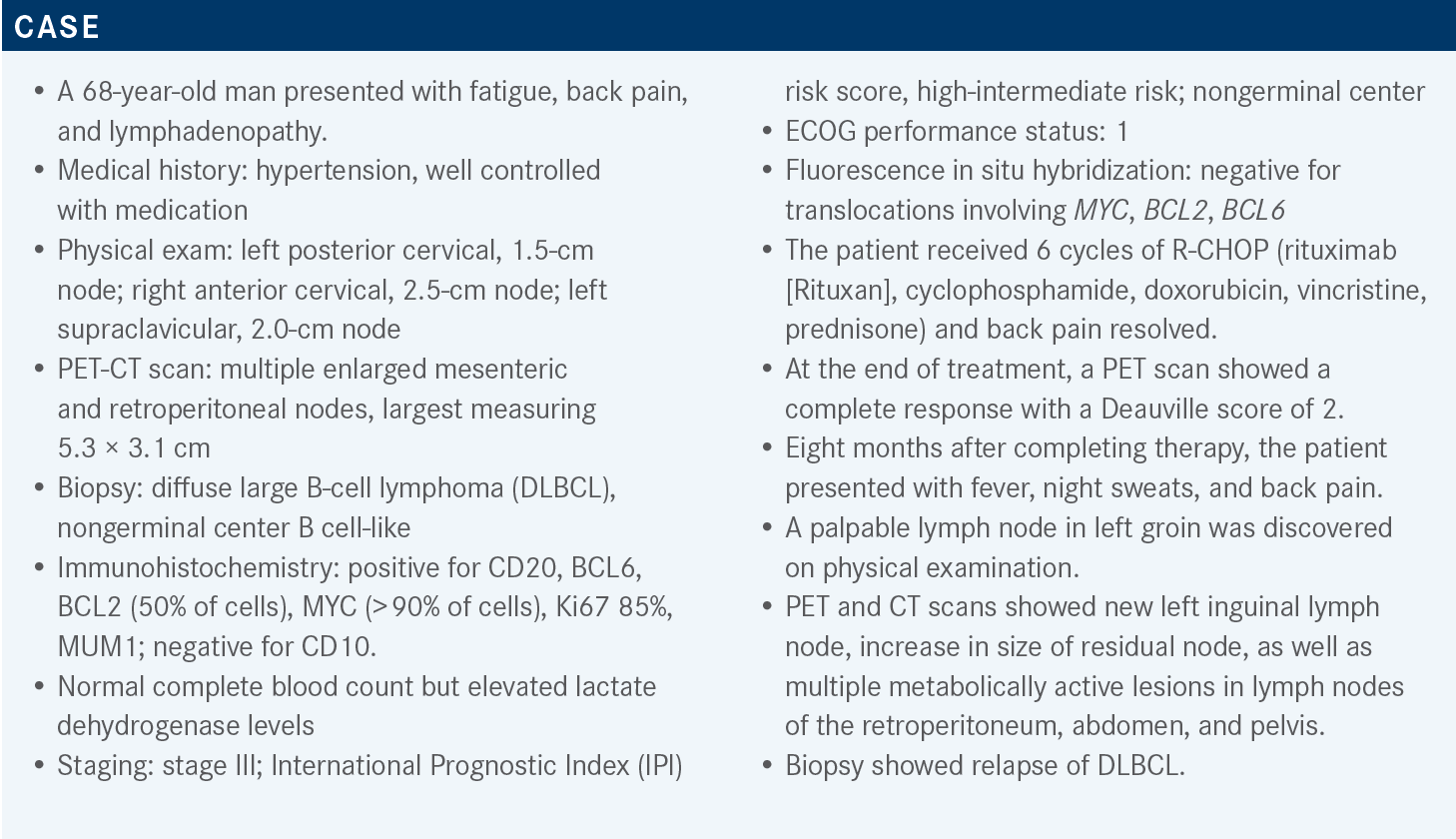
What options should be considered for such a patient at this juncture?
At this point, we face the more common salvage options. The preferred regimens are GemOx [gemcitabine (Gemzar) and oxaliplatin (Eloxatin)] with or without rituximab, polatuzumab vedotin-piiq [Polivy] with or without bendamustine [Bendeka] and rituximab, and tafasitamab-cxix [Monjuvi] plus lenalidomide [Revlimid]. These options are for patients who are not considered candidates for ASCT.1 The patient we are discussing is likely not a candidate for ASCT, because he [is unlikely to] respond to chemoimmunotherapy and [achieve] remission.
GemOx is something we’re all very familiar with, and I give it every 2 or 3 weeks. Polatuzumab vedotin [with or without] bendamustine, rituximab, [or both] is approved to treat large cell lymphoma [after more] than 2 lines of therapy.8 Polatuzumab is an antibody-drug conjugate that targets CD79b and has monomethyl auristatin E [MMAE] conjugated to it. MMAE is a common payload on a multitude of antibodies we use.
Tafasitamab is a CD19-targeted antibody, and lenalidomide is an immunomodulatory agent. This combination is approved to treat adults with relapsed or refractory DLBCL who are not eligible for ASCT.9
A key footnote is that we have very little data about whether tafasitamab[-cxix], loncastuximab tesirine-lpyl [Zynlonta], or any other CD19-directed therapy would have a negative effect on the efficacy of subsequent anti-CD19 CAR T-cell therapy.1 We’ll get more real-world data about these situations, even though most physicians prefer polatuzumab-piiq without bendamustine as a bridge to CAR T-cell therapy. [In light of] the adverse event [AE] profile of polatuzumab, the role of these other therapies and their impact on CAR T-cell therapy is something we’ll need to investigate. There are some preliminary data showing that loncastuximab has no real impact on the efficacy of CAR T-cell therapy, but unfortunately, we don’t have as much data for tafasitamab.
What data support the various recommended second-line therapies for transplant-ineligible DLBCL?
A [retrospective] study of R-GemOx [GemOx plus rituximab] looked at 196 patients with a median age of 72 years and a median of 1 prior line of therapy. A little over half of these patients [57%] were primary refractory. After a median follow-up of 22 months, the [ORR] was 38%, the CR rate was 33%, the median PFS was 5 months, and the median overall survival [OS] was 10 months.10
The combination of polatuzumab vedotin plus bendamustine and rituximab [BR] was compared with BR alone in a randomized study [NCT02257567], with 48 patients in each arm. [In the experimental arm], the median age was 67 years and patients had received a median of 2 prior lines of therapy. Three-fourths of these patients were primary refractory. After a median follow-up of 27 months, the [ORR] was 45%, the CR rate was 40%, the median PFS was 9.5 months, and the median OS was 12.4 months.11
The combination of tafasitamab plus lenalidomide was examined in the L-MIND study [NCT02399085]. [This study involved] 81 patients, with a median age of 72 years and a median of 2 prior lines of therapy. [A total of] 44% [of patients] would be considered refractory [to the last prior therapy]. After a median follow-up of 42.7 months, the [ORR] was 57.5%, the CR rate was 40.0%, the median PFS was 11.6 months, and the median OS was 33.5 months.12 It was [based on] these results that this combination was approved.9
How was the L-MIND trial designed? What additional data did it yield?
In the L-MIND trial, tafasitamab was given at a dosage of 12 mg/kg on days 1, 8, 15, and 22 on a 28-day cycle. During the first cycle, there was an additional loading dose given on day 4. Oral lenalidomide was given at a dosage of 25 mg daily on days 1 through 21 of a 28-day cycle. Beginning with cycle 4, tafasitamab was given on days 1 and 15. After a total of 12 cycles, lenalidomide was discontinued and patients could continue on single-agent tafasitamab. The study did have a modification of its eligibility criteria such that it excluded refractory patients while the study was ongoing, so there were some refractory patients [enrolled in the study], but [most] would be considered relapsed per our current definition.13,14
At baseline, patients’ [International Prognostic Index] score was evenly split between 0 to 2 and 3 to 5. Most patients [75%] had stage III or IV disease. Approximately half the patients [56%] had elevated [lactate dehydrogenase]. Additionally, 19% of the patients were considered to be primary refractory, 44% were refractory to the last prior therapy, and 11% had received a prior stem cell transplant. Finally, the distribution of subtypes germinal center B-cell– like [GCB], non-GCB, and unclassified or unknown was [10%, 25%, and 65%], respectively.12,13
After a little more than 35 months of follow-up, the CR rate was 40.0% and the partial response [PR] rate was 17.5%. Additionally, 16.3% of the patients had stable disease and another 16.3% had progressive disease. The ORR, combining the rates of CR and PR, was 57.5% and the median duration of response [DOR] was 43.9 months.12,13
Not surprisingly, the best DOR was seen in patients who achieved a CR [median DOR, not reached (NR); 95% CI, 43.9-NR]. Patients who achieved a PR had a very low DOR of 5.6 months [95% CI, 2.2-NR]. When these 2 groups were combined, the median DOR was 43.9 months [95% CI, 26.1- NR]. The median time to response was 2.1 months [range, 1.7-34.7] and the median time to CR was 6.8 months [range, 1.7-46.3].12 This [drug combination acts] a little more slowly than other cytotoxic agents, for which best responses are typically seen very early. Because of the mechanism of action of this combination, a [somewhat longer time is required] to get patients’ disease under control and to achieve the best response.
After a median of 33.9 months of follow-up, the median PFS was 11.6 months. The percentage of patients still in remission was 50% at 12 months and 46% at 18 months. After a median of 42.7 months of follow-up, the median OS was 33.5 months. The percentage of patients surviving was 74% at 12 months and 64% at 18 months. [In analyses of PFS and OS, as in the analysis of DOR], patients who achieved a CR had better outcomes [PFS, NR; 95% CI, 45.7-NR; OS, NR; 95% CI, 45.7-NR] [than did patients who achieved a PR (PFS, 7.4 months; 95% CI, 5.3-NR; OS, 22.5 months; 95% CI, 8.6-NR) or stable disease].12
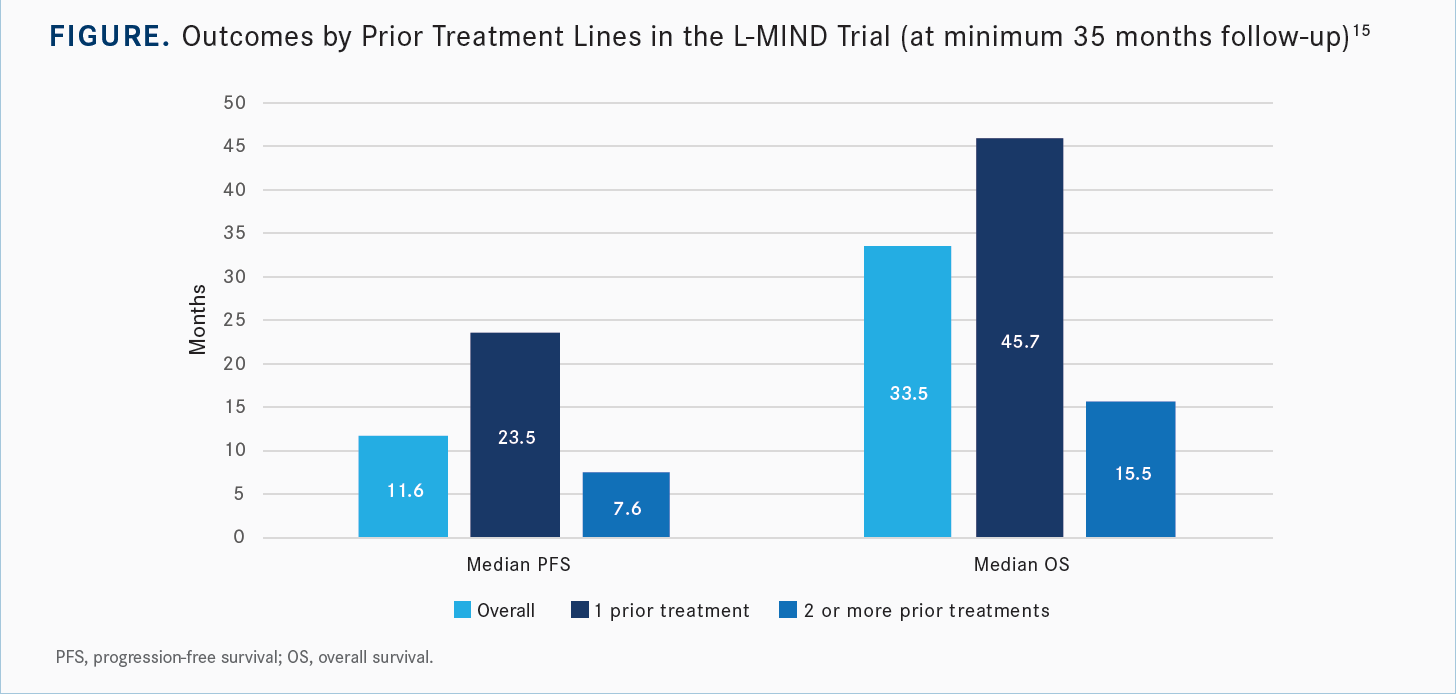
One key caveat that came from the L-MIND study was that the earlier you implement this treatment, the better the outcomes seem to be. Among patients who had received only 1 prior line of therapy, there was a higher percentage of patients with CR and with PR, a longer duration of response, and higher rates of PFS and OS relative to the group of patients who had received 2 or more prior lines of therapy [Figure15]. This is something to take into consideration, especially for frail patients or those who are unfit and unable to get to CAR T-cell therapy or ASCT.
The most common hematological AEs were neutropenia, anemia, and thrombocytopenia [of grades 1 to 4. These AEs affected 49%, 34%, and 31% of patients, respectively]. Other hematological AEs were leukopenia, febrile neutropenia, lymphopenia, and agranulocytosis. There were no grade 5 hematological AEs.13
The most common nonhematological AEs were rash, diarrhea, asthenia, cough, fever, peripheral edema, upper respiratory tract infection, and anorexia.13 Most of the AEs, including the hematological AEs, were consistent with what we see with single-agent lenalidomide.
Serious AEs occurred in 51% of the patients. Those thought to be treatment-related affected only 19%, as assessed by the investigators, and 12% of the patients had discontinuation of therapy [because of] AEs. There were 7 patients with AEs of special interest: [3 with tumor flare, 1 with basal cell carcinoma, and 3 with grade 3 allergic dermatitis]. Treatment-emergent AEs leading to death occurred in 13% of the patients [4 of 30]. One or more dose reductions of lenalidomide were required for 45.7% of the patients. Most patients [77.5%] were able to receive a lenalidomide dose of at least 20 mg daily over the duration of treatment, which is probably in line with the dosage most of us use.13,15,16
Upon discontinuation of lenalidomide, patients were able to continue treatment with single-agent tafasitamab. The AE profile of the combination was compared with that of single-agent tafasitamab, and the data showed that the incidence and severity of treatment-emergent AEs decreased during the tafasitamab monotherapy period. This is especially true of some of the AEs that are more consistently seen with lenalidomide. [When lenalidomide was discontinued], there was a complete resolution of thrombocytopenia and a reduction in the incidence and severity of neutropenia, anemia, diarrhea, asthenia, rash, and peripheral edema.14
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 5.2022. Accessed October 17, 2022. https://bit.ly/3zCxFro
2. FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma. FDA. April 1, 2022. Accessed October 17, 2022. https://bit. ly/3NtyisJ
3. FDA approves lisocabtagene maraleucel for second-line treatment of large B-cell lymphoma. Updated June 27, 2022. Accessed October 21, 2022. https:// bit.ly/3DvFT5E
4. Locke FL, Miklos DB, Jacobson CA, et al; All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. doi:10.1056/ NEJMoa2116133
5. Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629-639. doi:10.1056/NEJMoa2116596
6. Kamdar M, Soloman SR, Arnason JE, at al. Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): results from the randomized phase 3 TRANSFORM study. Blood. 2021;138(suppl 1):91. doi:10.1182/blood-2021-147913
7. Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294-2308. doi:10.1016/S0140-6736(22)00662-6
8. FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. FDA. June 10, 2019. Accessed October 17, 2022. https://bit.ly/2XEazLP
9. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed October 17, 2022. https:// bit.ly/3DTWfX2
10. Cazelles C, Belhadj K, Vellemans H, et al. Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory/relapsed diffuse large B-cell lymphoma: a real-life study in patients ineligible for autologous stem-cell transplantation. Leuk Lymphoma. 2021;62(9):2161-2168. doi:10.1080/10428194.2021.1901090
11. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
12. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. doi:10.3324/haematol.2020.275958
13. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
14. Salles G, Duell J, González Barca E, et al. Primary analysis results of the single-arm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Abstract presented at: 15th International Conference on Malignant Lymphoma; June 18-22, 2019; Lugano, Switzerland.
15. Düll J, Maddocks KJ, Gonzalez-Barca E, et al. Long-term analyses from L-MIND, a phase II study of tafasitamab (MOR208) combined with lenalidomide (LEN) in patients with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). J Clin Oncol. 2021;39(suppl 15):7513. doi:10.1200/JCO.2021.39.15_suppl.7513
16. Monjuvi. Prescribing information. Morphosys US Inc; 2021. Accessed October 10, 2022. https://bit.ly/3NseKF6
