Roundtable Discussion: Westin Evaluates Key Considerations for Primary Maintenance of Ovarian Cancer
Shannon N. Westin, MD and a group of peers discussed key consideration when approaching management of a patient with ovarian cancer.
Shannon N. Westin, MD
Director, Early Drug Development, Department of Gynecologic Oncology and Reproductive Medicine
Professor, Department of Gynecologic Oncology and Reproductive Medicine, Division of Surgery
Co-Director, Ovarian Cancer Moonshot Program
The University of Texas MD Anderson Cancer Center
Houston, TX


WESTIN: How do you discuss this with patients? What are your goals of therapy, the expected adverse events [AEs], how they’re going to be monitored, duration of therapy, all of that? Dr Mazharuddin, have you prescribed PARP inhibitors for patients in the primary maintenance setting?
MAZHARUDDIN: Yes, I have. The goals of therapy are mainly to prevent relapse or recurrence. I explain to them how it works, what to expect as far as AEs and everything, and we go from there. Usually, they’re just happy that it’s an oral treatment.
ALOBA: I tell them that it’s a way to try to prevent recurrence. Of course, we monitor the laboratory results a little bit more frequently in the beginning and counsel them about the AEs. We do more frequent follow-ups in the first 3 months. Then, if we know what AEs they’re experiencing from the drug, the visits are going to be less [frequent].
WESTIN: Yes, I think it’s a good point, and I’d be interested if others have experienced the same. But I do think that many times the bulk of the AEs from PARP inhibition seem to be early on. Once you get your patient on a good strategy for her, then often we can extend those visits out a little bit more.
Dr Reddy, how long do you keep your patients on their up-front PARP inhibitor? What’s your duration of therapy?
REDDY: I have used it with a patient for close to 1 year; finally we ended up stopping it because she didn’t like it, or the symptoms. Not so much cytopenias, just the fatigue.… After 10 or 11 months I ended up stopping it, and she’s still in remission.
WESTIN: Good. For a lot of the [Kaplan-Meier] curves, that big first split happens in that first year, maybe pushing in to 18 months. It does beg the question of how long should you continue the therapy. I think the olaparib [Lynparza] studies utilized the PARP inhibitor for 2 years.1,2 Niraparib [Zejula] studies have been 3 years.3,4 I think being mindful of how long we’re going to keep the patient on is important.
What about dose modifications? When you counsel them, do you talk to them about dose modifications early on, or do you kind of let things happen?
REDDY: In my patient, I didn’t discuss it at the time of starting [maintenance therapy]. I just want to get one thing at a time, say, “This is what it is, let’s get started.” I didn’t discuss the dose [at that point].
MOSCOL: I don’t go into too much detail, either. I just think that when you’re doing the informed consent you need to relay that hematologic toxicity is expected, and eventually we’ll need to hold, or dose reduce, or improve supportive care. It’s overwhelming if you want to go over those details all at once.
WESTIN: I agree with you, Dr Moscol. I don’t like to do a ton of detail. I have started level-setting a little bit with my patients, where I’ll say, “We’re going to start you at this level, but this might not be the right one for you, and we’re going to find your sweet spot of dosing.” And let them know that it’s OK to stop sometimes. I want them to report those AEs.
I feel like patients think, “If I’m not getting the maximum dose of chemotherapy, or the maximum dose of this, or if I’m not right on schedule, I’m going to have worse outcomes.” I think letting them know that that’s not the case with these drugs, and sometimes we do need to make adjustments, and it’s OK, is important.
MOSCOL: [Outcomes based on dose reductions] would be very interesting data. In breast cancer, for example, ribociclib [Kisqali] has very interesting data showing that even if you dose reduce by 1 level, the Kaplan–Meier curves looked exactly the same.5 So, it was reassuring to know that even when you are cutting back on some of the dose, you still weren’t losing efficacy. I don’t know if they have any of that for PARP inhibitors in ovarian cancer, but that would be quite [helpful].
WESTIN: I’ve seen some early exploratory [analyses] looking at that. The ATHENA trial [NCT03522246] that was just presented at the 2022 American Society of Clinical Oncology Annual Meeting, which was looking at rucaparib [Rubraca], showed dose intensity and benefit with dose reductions.6 They saw consistent benefit, even amongst those patients that have a relatively lower dose intensity due to a dose reduction. I think [clinical trial investigators are] starting to realize that we want to see those data, because we know that some of these patients are going to need to be dose reduced. It’s important for us, as we’re counseling those patients.
What about when to take the medications? Obviously, for olaparib, it’s morning and night, so you don’t get to do any manipulation of when patients take medicines. Do you have any specific way you dose niraparib?
KHADE: I typically just counsel the patient on making sure that they take it at the same time every day, whether it’s in the morning or at night.
WESTIN: That’s exactly how I feel, but I will say that if I have patients that are getting some nausea with niraparib, then I will push it to the evening time. They can take an antiemetic and go to bed, and sleep through most of the nausea. That is a strategy that I’ll sometimes use with niraparib, if I’ve got a patient who’s struggling with nausea. It sometimes can help with fatigue, too. If they take it in the evening, they’re not quite as tired during the day. They sleep off the worst of the fatigue.
WESTIN: How do you feel about these data? I don’t know if you had seen the PRIME data before.4 Does this make you feel more comfortable potentially starting niraparib at 200 mg for the appropriate patient?
KHADE: I haven’t used it with the individualized starting dose, but seeing the data, I would feel comfortable doing that. The [efficacy] was still sufficient, whether you were using the 200-mg or 300-mg dose, when dose reduced for patients with lower body weight or low platelets [From the Data4]. I think [thrombocytopenia] is one of the main toxicities that we see, so anything that we use to prevent that, while still maintaining efficacy, is good. So, yes, I would feel comfortable dose reducing.
WESTIN: Yes, and it is profound. I just had a [patient] I started on niraparib who went down to a platelet count of 10 x 109/L. When it happens, it catches your eye. So, if there’s any way to reduce the incidence of that, we want to do it. I think we’re getting more and more consistency, that it’s safe and effective.
REDDY: A patient I used it with had a large tumor, and I used it based on the platelets and weight. Her [platelet] counts were not an issue, but…she could not tolerate it, because of fatigue. So, I switched her to bevacizumab [Avastin], even though she’s BRCA-positive, because she could not tolerate [niraparib].
WESTIN: I think our goal, of course, is to try to give the patients the best therapy possible, but tolerance can be an issue. Frequent use of dose interruption and dose reduction is critical, because each patient is going to be fairly unique. I definitely have had some patients—and this goes both ways—that weren’t tolerating one [PARP inhibitor], and I switched to another, just to see if we had a better safety profile, and sometimes you can get away with that, too.
REDDY: I don’t know how good bevacizumab maintenance is on BRCA-positive patients.
WESTIN: Patients with BRCA-positive disease do better in general, so there are some data with bevacizumab [suggesting] that they’ll get benefit. You cannot compare cross-trial, but…when you look at median [progression-free survival], the patients definitely go longer without progression when they’re treated with a PARP inhibitor.
What do you think are the most challenging AEs? I’ve heard you talk about [blood cell] counts and a little nausea, and fatigue was another thing that was brought up. Of those things that you’re seeing commonly with these PARP inhibitors, what challenges you the most? What’s making you discontinue?
MAZHARUDDIN: I’d say the [blood cell] counts. I can manage nausea with good doses of ondansetron [Zofran]. I’m comfortable with all of those things. But the counts can get a bit difficult, especially with thrombocytopenia.
WESTIN: Especially with thrombocytopenia; we can transfuse, but only in desperate times. There’s not a lot you can do medically. I find fatigue the hardest because I don’t have anything good to give them. We don’t have a drug that we can reverse fatigue with, so I feel like fatigue, for me, is the most common cause of needing to dose interrupt and dose reduce.
I think that’s a critical piece. If you just dose reduce when the patient’s having fatigue, it’s going to be hard for her to improve. You want to give them a break and let them recuperate and get back up to their baseline, or at least close to their baseline, before we restart.
I’ve had some patients with—[and] I’ve seen this reported in a few of the studies—insomnia, some sleep disturbance and things like that. I think that can be a little bit of a struggle, especially if they’re already fatigued, and then they’re not sleeping well. It certainly can be a troublesome one.
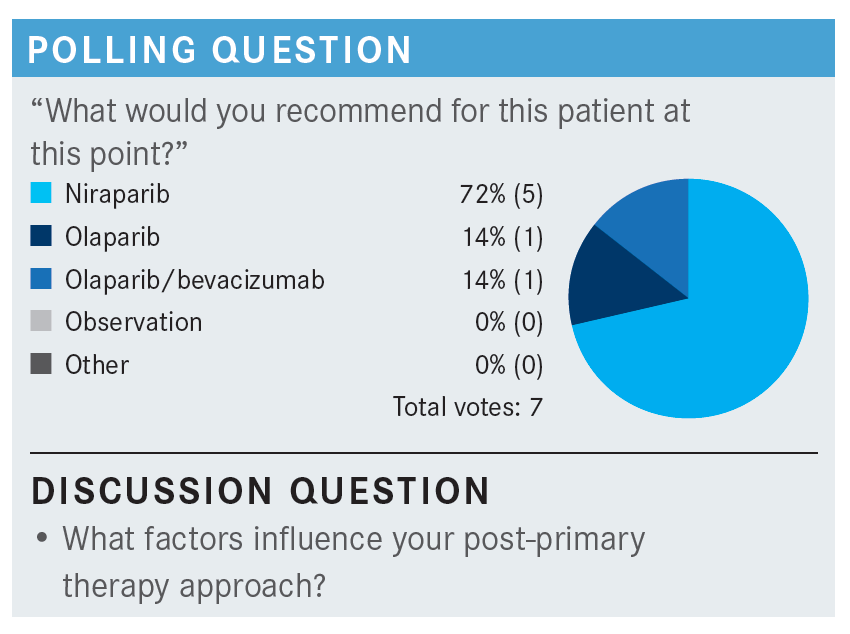
WESTIN: What would you give her for chemotherapy and how do you make that decision?
ALOBA: I would give her carboplatin/paclitaxel/bevacizumab, and then I would give her niraparib as maintenance.
WESTIN: So, you would switch her off the bevacizumab and just do the niraparib on its own?
ALOBA: Yes.
WESTIN: I love those PAOLA-1 [NCT02477644] data too, though, [for the] combination [of olaparib and bevacizumab for maintenance].2 When you look at the median of time that the patients with HRD stayed on, it’s 37 months. Pretty impressive.
It’s hard to compare it to PRIMA [NCT02655016], though, because it was a bit of a [worse-performing] population, but I do wonder if there’s something [favoring] that combination in that population with HRD.3 But I often will switch patients to niraparib as well, when they don’t want to keep doing injections or infusions and want to just transition to a pill.
Is there anybody you wouldn’t use bevacizumab in? Or is there anybody who doesn’t use bevacizumab with chemotherapy? I’m a little more selective. I don’t use it for everybody. Dr Khade, do you use any bevacizumab in the up-front setting for these patients?
KHADE: Typically not. Typically, we just use the combination of carboplatin/paclitaxel. [Bevacizumab] is an option, I just personally have never used it.
WESTIN: Anybody else use it?
REDDY: Unless there is a true contraindication, my default is with bevacizumab. The only thing is the dose; [for] colorectal cancer it is 5 mg/kg, but we use 15 mg/kg [in ovarian cancer, so I] get a little concerned.8 I start with 10 mg/kg, and then slowly increase if they tolerate it. It [sometimes] scares me to start at 15 mg/kg.
WESTIN: It is interesting how different all the doses are across all the different cancer types, or even between studies in ovarian cancer. The European study, the ICON7 study [ISRCTN91273375], [used] 7.5 mg/kg.9 I do agree; it’s unusual.

REDDY: So when you use it, do you use mostly with 10 mg/kg, Dr Westin?
WESTIN: I do. Sometimes, I’ll use 15 mg/kg, depending on the patient. But we’ve had pretty good results and pretty good toxicity profiles, so I don’t worry too much about it.
WESTIN: For this patient specifically, what factors influence your choice of maintenance? Do you think there’s a difference in efficacy between these agents? Is that something that you look at, or is every [agent] the same?
The data all look pretty consistent, right? The question I have, that we won’t have from PAOLA-1, is the combination of olaparib and bevacizumab over just the PARP inhibitor alone.2 That would [have] been a nice arm to have in that study, to know if bevacizumab adds something for the population that has a BRCA mutation or HRD. That’s always a question in my mind.
Are there any AEs that they’ve had during chemotherapy that will make you go in one direction or the other? Do you consider comorbidities, like hypertension?
MOSCOL: It depends on how bad the hypertension is, right? But certainly with bevacizumab, I’ll be a little more concerned if you’re trying to use that, you have to watch for nephrotic syndrome [and] edema. If you have a previous history of CAD [coronary artery disease]—this patient is young, but for older patients, I pay attention to it. Stroke, transient ischemic attack, CAD; I would just consider those contraindications.
WESTIN: Yes, I think just to be mindful too, niraparib can cause elevation of blood pressure. It’s only in about 20% of patients, but it is something to be mindful of. But, conversely, it has less interaction with other drugs, so if you do have a patient with polypharmacy, sometimes that one is a little bit easier. So those are some of the things that we use in our clinic as we’re deciding.
REFERENCES:
1. Moore K, Colombo N, Scambia G, et al.Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505. doi:10.1056/NEJMoa1810858
2. Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med.
2019;381(25):2416-2428. doi:10.1056/NEJMoa1911361
3. González-Martín A,Pothuri B, Vergote I, et al; PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med.2019:381(25):2391-2402.doi:10.1056/NEJMoa1910962
4. Li N, Zhu J, Yin R, et al. Efficacy and safety of niraparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer using an individualized starting dose (PRIME Study): A randomized, double-blind, placebo-controlled, phase 3 trial (LBA 5).Gyn Oncol. 2022;166(suppl 1):S50-S51. doi:10.1016/S0090-8258(22)01298-7
5. Hart LL, Bardia A, Beck JT, et al. Impact of ribociclib (RIB) dose modifications (mod) on overall survival (OS) in patients (pts) with HR+/HER2-advanced breast cancer (ABC) in MONALEESA(ML)-2.J ClinOncol. 2022;40(suppl16):1017-1017. doi:10.1200/JCO.2022.40.16_suppl.1017
6. Monk BJ, Parkinson C, Lim MC, et al. ATHENA–MONO (GOG-3020/ENGOT-ov45): A randomized, double-blind, phase 3 trial evaluating rucaparib monotherapy versus placebo as maintenance treatment following response to first-line platinum-based chemotherapy in ovarian cancer.J Clin Oncol. 2022;40(suppl17):LBA5500-LBA5500. doi:10.1200/JCO.2022.40.17_suppl.LBA5500
7. MyChoice CDx.Myriad Genetics. Myriad Oncology. Accessed October 20, 2022. https://bit.ly/3UJqTbo
8.Avastin. Prescribing information. Genentech, Inc; 2022. Accessed November 9, 2022. https://bit.ly/3tmjsLj
9.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med.2011;365(26):2484-2496. doi:10.1056/NEJMoa1103799

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More