Shah Explores Treatment Options for Patients With Advanced HER2+ Gastric Cancer
During a live event, Manish A. Shah, MD, discussed the case of a 65-year-old man presented with new onset fatigue, upper abdominal pain that worsened with eating, and unintentional weight loss.
Manish A. Shah, MD
Director, Gastrointestinal Oncology Program
Professor of Medicine
Weill Cornell Medicine
Chief, Solid Tumor Service
Codirector, Center for Advanced Digestive Disease
NewYork-Presbyterian
New York, NY

Targeted OncologyTM: What do you think are the most important data regarding the combination of pembrolizumab and trastuzumab for treating patients with HER2-positive unresectable or metastatic gastric or gastroesophageal cancer?
SHAH: KEYNOTE-811 [NCT03615326] is based on a [prior] phase 2 study [NCT02954536] run by Yelena Y. Janjigian, MD. In that study there was a 91% overall response rate [ORR], so that led to a global phase 3 study of chemotherapy.1-3 [The investigators] used FP or capecitabine plus oxaliplatin [CAPOX]. That way it was an every-3-weeks schedule with trastuzumab and they [randomly assigned] patients to placebo or pembrolizumab.2,3 The primary outcome was OS [overall survival]. The second primary end point was PFS [progression-free survival], and ORR was a [key] secondary end point.
In the first interim analysis they were looking at ORR in the first 260 patients, and the total study size was 692, so not even half of all the patients were enrolled.2 They were looking at the first 260 [patients], and they had a superiority bound with a 1-sided P value of .002. As typical for gastric and GEJ cancers, most [of the patients] were male, and [in terms of geographic location], 31% were in Australia plus a Western population, 30% in Asia, and then the rest of the world was about 40%. Most patients had true stomach cancers [72% in the experimental arm and 68% in the placebo arm]; most had an intestinal [histological subtype]. Most patients with HER2-positive disease are going to be PD-1 positive as well, and [so] 85% to 89% [were PD-1 positive]. Most patients’ HER2 status was IHC 3+. IHC 3+ gastric tumors are driven by HER2, so even without pembrolizumab, I have several patients who are doing well 3 to 4 years down the line with targeting the HER2 aspect here. Most patients got CAPOX, very few got FP.
The patients who were [randomly assigned] to receive chemotherapy plus trastuzumab plus pembrolizumab had a very high response rate.2 The ORR was around 74.4%, but about 97% had some decrease in their tumor volume. Radiographically, 11% had a clinical complete response [CR], which is quite tremendous. The duration of response [DOR] was significant; it was a median of about 10 months. So if you’re responding to this regimen, you’re going to respond well. But in the placebo arm, the DOR was 9.5 months, so it’s also very active. The disease control rate was 96% [with pembrolizumab] vs [89% in the placebo group]. Trastuzumab has a lot of activity, but I think adding pembrolizumab probably does have more activity, and based on that, the FDA did approve pembrolizumab in the first-line setting.4
Toxicities were similar in terms of the serious adverse events [AEs], about 30% in both arms.2 The most common toxicities of grade 3 to 5 were anemia and diarrhea. Those are the most common but [they were] actually less than 10% for both arms. Generally, it was a pretty well-tolerated regimen. In terms of the immune-specific toxicities, infusion reactions [of grade 3 or higher] rarely [occurred], pneumonitis [of grade 3 to 5 occurred at a rate of 1% in the treatment arm and not at all in the placebo arm], [and] colitis of [grade 3 to 5 occurred at a rate of 3% in the treatment arm and 1% in the placebo arm]. I have to be honest, in my practice, I see more grade 3 and 4 [immune-mediated] toxicity than is reported here, which is less than 5%. But in this randomized study, pembrolizumab doesn’t seem to add that much toxicity.
What is the role of fam-trastuzumab deruxtecan (Enhertu) in treating patients with HER2-positive gastric cancer?
This is an antibody-drug conjugate, and there are 3 components.5 There’s the humanized anti-HER2 monoclonal antibody, which is very similar to trastuzumab, but the payload is the drug that’s linked to the trastuzumab antibody through a linker, and the payload in this case is a topoisomerase I inhibitor. It’s an exatecan derivative, and then the linker is a tetrapeptide-based cleavable linker. What happens is that the drug binds trastuzumab, gets internalized, and then the topoisomerase I inhibitor gets freed and then can go into the nucleus and bind [and inhibit] topoisomerase I. The other thing is the drug-to-antibody ratio, or DAR. For the other HER2-targeted antibody-drug conjugate, it’s T-DM1, which is trastuzumab with emtansine [Kadcyla], and for that one, the DAR is 4.6 This drug-antibody conjugate has a DAR of 8, [which] is pretty high.5 So you’re getting a lot of drug in with the antibody.
The DESTINY-Gastric01 study [NCT03329690] is a study [that randomly assigned patients to receive] trastuzumab deruxtecan at 6.4 mg/kg vs physician’s choice, [being] chemotherapy—irinotecan [Camptosar] or paclitaxel. It was a 2:1 randomization.7 It was a small study of 188 patients. The arms were well balanced in terms of age and [sex]. It was a study done in Asia only, so most of the patients were from Japan but there were some from South Korea as well. Most patients had IHC 3+ [HER2 expression] but not all. Some tumors were quite big and bulky, but about half the patients’ tumors were less than 5 cm. One hundred percent of patients had prior trastuzumab therapy, so this was truly a trastuzumab-refractory population.
In this trastuzumab-refractory population…trastuzumab deruxtecan had a 51% ORR. It actually went down to 43% after central review, but it’s still a high response.6 Chemotherapy had a 14% response, and that’s about right for chemotherapy. If you use paclitaxel and ramucirumab [Cyramza], your ORR is [28%].8 Some patients had a CR, about 10%, which is quite amazing in this setting.6
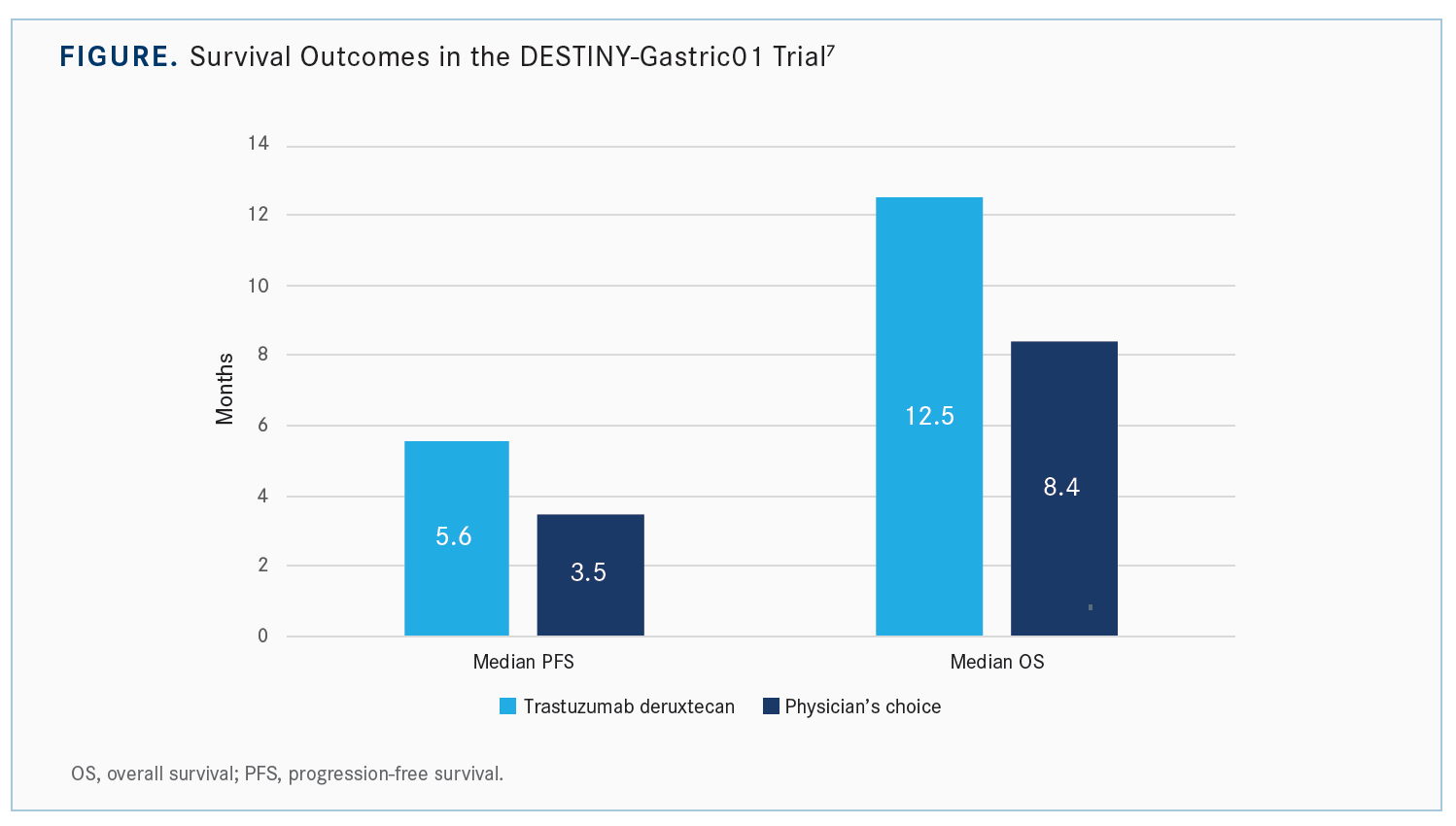
The PFS [Kaplan-Meier] curves for trastuzumab deruxtecan show [approximately] a 2-month improvement in median PFS [5.6 months vs 3.5 months with chemotherapy], but the tail on the curve is impressive.7 So 30% of patients are without progression at a year with trastuzumab deruxtecan vs none who got chemotherapy. Initially, maybe half the patients have only marginal benefit, but the tail of the curve is quite impressive. About 25% have quite durable responses going out to 1.5 to 2 years. In terms of median OS, it is much better, [approximately] 12 months if you use trastuzumab deruxtecan vs 8 months with chemotherapy [Figure7]. The [Kaplan-Meier] curves split pretty early, and they stay split.
If you look more carefully at who was responding, for the most part it was independent of the number of [prior] lines of therapy, in terms of the region, [sex], or anything else.7 It’s interesting, though, in the HER2 IHC 3+ group, the benefit was quite significant. In the HER2 IHC 2+ [group with only] 44 patients, chemotherapy did about as well as trastuzumab deruxtecan. The benefit was primarily in HER2 IHC 3+, not HER2 IHC 2+ FISH positive. The benefit was equal in gastric or GEJ tumors and perhaps slightly better in diffuse gastric cancer. If you had a total gastrectomy and you got the trastuzumab deruxtecan, you did much better. The numbers are small [n = 31 patients], so I don’t know if that is a fluke, but the confidence limits don’t overlap, so it may be the most significant thing.
In terms of toxicity with chemotherapy vs trastuzumab deruxtecan, chemotherapy is generally pretty well tolerated: [23% grade 3 or 4] anemia, [24% grade 3 or 4] neutropenia.9 With trastuzumab deruxtecan, the toxicity is a little bit higher, [51% had grade 3 or 4] anemia [and 38% had grade 3 or 4] neutropenia. It does have a little more toxicity than you would see with chemotherapy.
Pneumonitis is probably the most worrisome AE. At the initial experience, there were a handful of [patients] who had grade 5 [pneumonitis], but I think the point is that if you identify it early, at the initial onset of shortness of breath or if you have some findings on a CT scan, you can manage the pneumonitis much better with steroids, holding the drug, and so forth.9
REFERENCES:
1. Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821-831. doi:10.1016/S1470-2045(20)30169-8
2. Janjigian YY, Kawazoe A, Yanez PE, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/ GEJ) cancer: initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol. 2021;39(suppl 15):4013. doi:10.1200/JCO.2021.39.15_suppl.4013
3. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727-730. doi:10.1038/s41586-021-04161-3
4. FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer. FDA. May 5, 2021. Accessed November 7, 2022. https://bit.ly/3Ekl6DT
5. Enhertu. Prescribing information. Daiichi Sankyo Inc; 2022. Accessed October 21, 2022. https://bit.ly/3t6Xk7F
6. Kadcyla. Prescribing Information. Genentech; 2022. Accessed October 21, 2022. https://bit.ly/3fDuLff
7. Yamaguchi K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: final overall survival (OS) results from a randomized, multicenter, open-label, phase 2 study (DESTINY-Gastric01). J Clin Oncol. 2021;39(suppl 15):4048. doi:10.1200/JCO.2021.39.15_suppl.4048
8. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. doi:10.1016/ S1470-2045(14)70420-6
9. Shitara K, Bang YJ, Iwasa S, et al; DESTINY-Gastric01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419-2430. doi:10.1056/NEJMoa2004413
