Chao Discusses Stratifying and Treating Patients With Graft-Vs-Host Disease
During a Targeted Oncology case-based roundtable event, Nelson Jen An Chao, MD, discussed risk management and treatment of patients with steroid-refractory acute graft-versus-host-disease as well as chronic graft-versus-host disease.

Nelson Jen An Chao, MD
Donald D. and Elizabeth G. Cooke Cancer Distinguished Research Professor
Chief, Division of Cell Therapy in the Department of Medicine
Duke Cancer Institute
Duke University School of Medicine

Targeted OncologyTM: How do you stage and grade acute GVHD [aGVHD]?
CHAO: There are multiple ways of staging aGVHD. The Mount Sinai Acute GVHD International Consortium [MAGIC] has 2 staging systems, organ staging and overall clinical staging, which are based upon the most severe target organ involvement. The organ staging goes from 0 to 4 and it’s straightforward, focusing on the skin, liver, and upper GI [gastrointestinal] and lower GU [genitourinary]. It hasn’t changed much; the main change in the last 10, maybe 15 years is the introduction of the upper GI GVHD, which includes nausea, vomiting, or anorexia. If you have those, you’re at stage 1 and then the output is measured. It used to be just volume in the Glucksberg criteria in the past, but I think with the more current staging, the episodes are added into the GI staging as well.1
From the stage, the overall grade is developed, so grade 0 is none and then grade 1 is 2 skin symptoms without symptoms in any other organ. Grade 2 is skin symptoms with grade 1 symptoms of the liver or the gut, and so forth, and this gives one the ability to discuss GVHD staging and grading across trials.
[In 2012, investigators looked at a different system] to stratify the risk based on organ involvement and the number of organs involved. They looked at the grading of the organs of about 1600 patients and stratified based on standard vs high risk. For example, a single organ or just GI or liver with 2 organs was considered a standard risk. The high-risk group had very high single-organ magnification, like grade 4 skin, grade 3 to 4 GI, or grade 1 to 4 liver or 2 organs. If there are 3 organs, it is grade 1 to 3 of skin plus grade 3 to 4 of the gut or the liver. What they were able to do with this was by looking at this risk score, determine what the probability of response and probability to survival was.2
High-risk GVHD is associated with lower responses to steroids and higher treatment-related mortality. Standard-risk responders, both partial and complete responders, did much better than those at high risk. The probability of treatment-related mortality also is much higher for high-risk compared with standard-risk patients. This probably reflects the total amount of immunosuppression of these patients, as high-risk patients have a much more advanced-stage disease.

How do you approach treatment for a patient with steroid-refractory GVHD?
This patient has steroid-refractory resistance, which usually progresses within 3 to 5 days. If you don’t get better within 5 to 7 days or after 20 days of immunosuppressive steroids, you’re not improving as expected. You’re improving, but you’re not much better. With steroid dependence, when you try to taper the patient, you get recurrence of aGVHD, and steroid intolerance is usually associated with unacceptable toxicity.3
The NCCN [National Comprehensive Cancer Network] has suggested agents for steroid-refractory GVHD. Ruxolitinib [Jakafi] is approved in the acute setting, but I think the key piece is clinical trials. We have lots of drugs, including alemtuzumab [Campath], and the approval for most of the drugs was based on studies with 30 patients in general that showed some response.…In the chronic setting, ruxolitinib, belumosudil, and ibrutinib [Imbruvica] are approved.4
What is the evidence for using ruxolitinib in patients with steroid-refractory aGVHD?
Ruxolitinib is pretty good at inhibiting STAT signaling, which basically decreases the signaling of interferon and IL-17, both of which are proinflammatory cytokines. The phase 2 REACH-1 study [NCT02953678] enrolled 71 non-[randomly assigned] patients with aGVHD, who were treated with 5 mg ruxolitinib twice a day, and the primary end point was overall response at day 28.5,6
The overall response rate [ORR] at day 28 was 54.9%, with 26.8% achieving a complete response [(CR). On the whole,] the ORR was pretty good at 73% [CR, 56.3%]. The median time to response was 7 days [range, 6-49 days] and the duration of response [DOR], with more than 6 months of follow-up, was 345 days. There were [35] deaths [49.3%] and the nonrelapse mortality was fairly high at 44%, but the median OS [overall survival] for day 28 responders was not reached.7
These 71 patients went on to the phase 3 REACH2 study [NCT02913261]. This was a prospective randomized trial with 294 patients enrolled in a 1:1 randomization to ruxolitinib 10 mg twice a day or the best alternative therapy. It’s an interesting design because the comparator included 10 different drugs [Figure7].
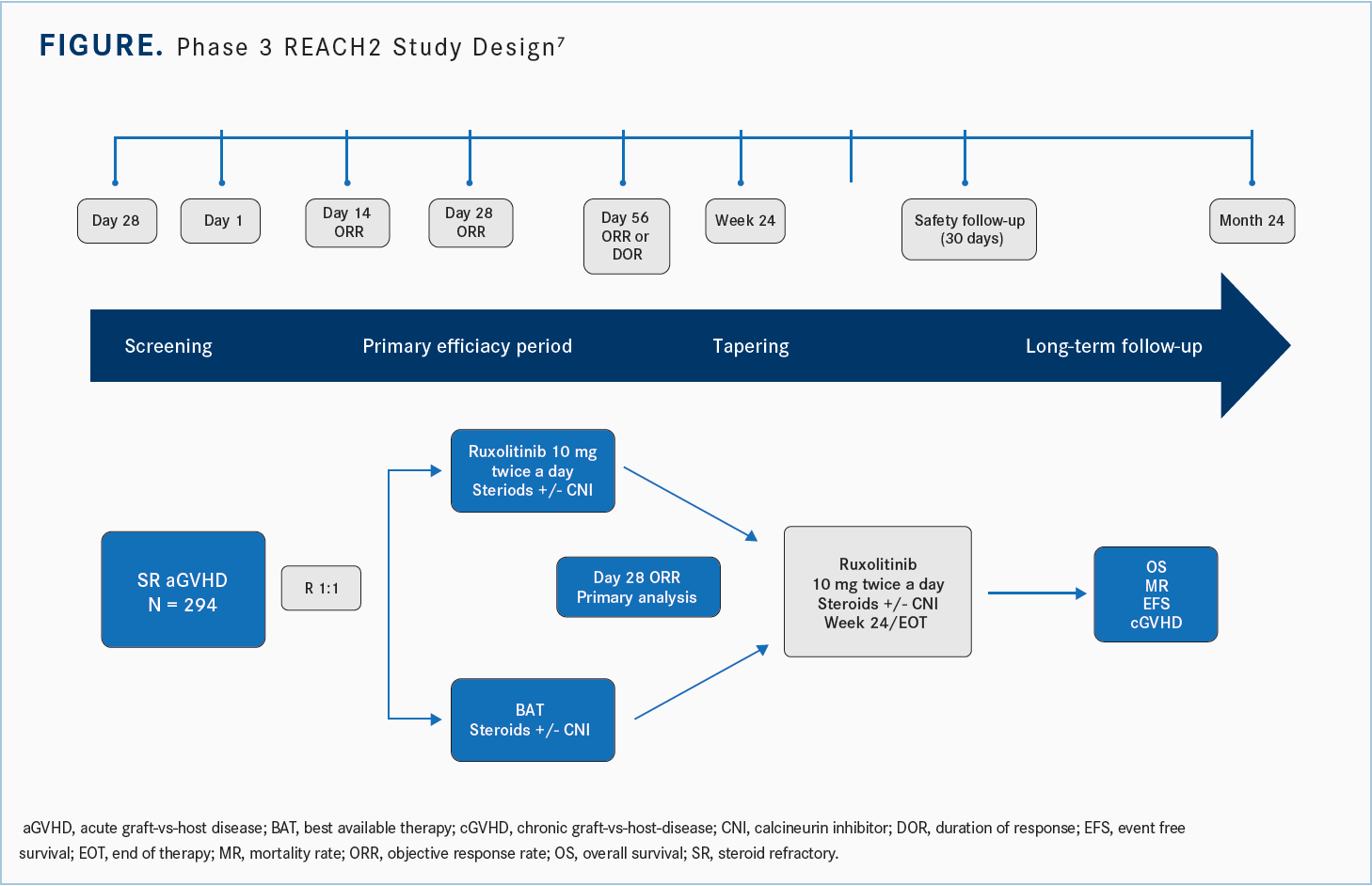
What were the results of REACH2?
[In the study,] you could use antithymocyte globulin, extracorporeal photopheresis [ECP], mesenchymal stromal cells, low-dose methotrexate, mycophenolate mofetil [MMF], everolimus [Afinitor] or sirolimus [Rapamune], etanercept [Enbrel], and infliximab [Remicade]. The numbers of patients enrolled in any one of these best alternative therapies was essentially driven by the institutions which enrolled these patients.6
The primary analysis was done on day 28 and these were patients who had severe refractory aGVHD grade 2 vs grade 3 and 4 and [who were aged] greater than 12 [years]. They had myeloid engraftment, but…were excluded if they had active infections or severe organ failure, if they had failed prior allogeneic hematopoietic stem cell transplant [HSCT] in the last 12 months, or if the disease had relapsed.
The study did allow for crossover, so if patients in the best alternative therapy group had not had a response by day 28 they could cross over to ruxolitinib. Then they looked at OS, event-free survival [EFS], and chronic GVHD [cGVHD].
The primary end point of the study was ORR at day 28, which was 63% for ruxolitinib compared with 39% in the control arm. Both partial responses [PRs] and CRs were higher with the ruxolitinib over the control arm, and durable responses were almost double for ruxolitinib [vs] the control on day 56 [odds ratio, 2.38; 95% CI, 1.43- 3.94; P < .001].8
When stratified by grade of disease, the odds ratios were significantly in favor of ruxolitinib. The odds ratio for the full analysis set was 2.64 [95% CI, 1.65-4.22]; for grade 2 it was [2.96 (95% CI, 1.30-6.76)], grade 3 was [2.15 (95% CI, 1.10-4.20)], and grade 4 was [3.76 (95% CI, 1.24-11.38)].8
The percentage of patients who lost response was much higher in the control group—over 40% compared with 10% to 15% in the ruxolitinib group. So patients taking ruxolitinib were much less likely to lose their response.8
Another interesting result was the shift in organ staging. Not every patient had a CR, but there was a shift to lower stages with ruxolitinib compared to the best alternative therapy. Overall, stage shifting downwards with ruxolitinib was better than [with] best alternative therapy.8
The failure-free survival [FFS] was quite significantly in favor of ruxolitinib. The percentage of patients without treatment failure was better with ruxolitinib [vs] the control. Obviously, this is not the answer to all of it and some patients are still failing therapy.8
Another cautionary point is that the toxicities are somewhat problematic with ruxolitinib. Thrombocytopenia is a known problem, and infections with CMV were perhaps a little higher than the control. But overall, the other adverse effects [AEs] were not very different between the 2 groups.8
Considering the REACH2 data, what do you use as first-line therapy for steroid-refractory aGVHD?
Ruxolitinib is an approved drug and I think it’s quite reasonable. I think many of us still have clinical trials ongoing or patients with steroid-refractory aGVHD, and if there’s equipoise in the studies, the clinical trials could fall under the other section [of treatment decisions].
What are the therapeutic options for this patient?
The phase 3 REACH3 trial [NCT03112603] investigated the use of ruxolitinib in the chronic setting. The most remarkable part of this trial was the total number of patients: 329 patients enrolled with 1:1 randomization to 10 mg of ruxolitinib twice a day compared with best alternative therapy.
The best alternative therapies included ECP, low-dose methotrexate, MMF [mycophenolate mofetil], everolimus or sirolimus, infliximab, rituximab [Rituxan], pentostatin [Nipent], and imatinib [Gleevec]. The study did allow crossover to ruxolitinib after cycle 7, day 1, if the patient hadn’t responded. The time for the initial response was 24 weeks, which looked at the overall response with CR plus PR, and the secondary end point was FFS and patient’s symptoms at week 24.9
What were the efficacy results of REACH3?
The response rates were higher for ruxolitinib, about 50% for ruxolitinib compared with 25% for the control on week 24. The best overall responses were 76% with both CRs and PRs compared with 60% with the best alternative therapy.9
The FFS was statistically significant for ruxolitinib, significantly better than the control arm, with the median FFS greater than 18.6 vs 5.7 months [HR, 0.37; 95% CI, 0.27-0.51; P < .001]. The DOR was also much better for ruxolitinib. The Kaplan-Meier median, estimating the probability of FFS at 6 months, was not reached for ruxolitinib compared with the control, which was 6.24 months.9
Are there other therapies to consider for this patient population?
In July of last year, the FDA approved belumosudil for [patients with] cGVHD after failure of at least 2 prior lines of systemic therapy. That’s the label indication, but the toxicity profile for the drug was significantly quite favorable and patients seemed to tolerate this very well. [These data are from] a randomized open-label trial with 65 patients.10
This [approval] was based on the ROCKstar study [NCT03640481], which was open label with 2 different doses at arm 1 with 200 mg a day and arm 2 with 200 mg twice a day. There’s no difference in the outcomes, so the recommended dose is once a day. The median time from cGVHD diagnosis was 25 months; 48% [of patients] had 4 more organs involved. The median number of prior lines of therapy was 3 and more than three-fourths [of patients] were refractory to the last therapy.11
There were clearly significant AEs for these drugs, but most would not make it difficult for the patients to tolerate this drug. Overall, it was quite well tolerated.
The ORR in the 200-mg arm was about 50% and the overall response occurring within the 12 months of therapy was quite high for these patients. FFS was 73% and the mean change was quite good, with about 50% of the patients improving. Overall, the clinically significant improvement from baseline was about 40% of these patients. So, good responses in a drug that was well tolerated.
REFERENCES
1. Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10. doi:10.1016/j.bbmt.2015.09.001
2. MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. doi:10.1016/j.bbmt.2015.01.001
3. Schoemans HM, Lee SJ, Ferrara JL, et al; EBMT (European Society for Blood and Marrow Transplantation) Transplant Complications Working Party and the “EBMT-NIH (National Institutes of Health)-CIBMTR (Center for International Blood and Marrow Transplant Research) GvHD Task Force. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401-1415. doi:10.1038/s41409-018-0204-7
4. NCCN. Clinical Practice Guidelines in Oncology. Hematopoietic cell transplantation (HCT), version 2.2021. Accessed April 23, 2021. https://bit.ly/3z4IyTn
5. Chao N. Finally, a successful randomized trial for GVHD. N Engl J Med. 2020;382(19):1853-1854. doi:10.1056/NEJMe2003331
6. Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, von Bubnoff N. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391-402. doi:10.2217/imt-2017-0156
7. Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749. doi:10.1182/blood.2020004823
8. Zeiser R, von Bubnoff N, Butler J, et al; REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. doi:10.1056/NEJMoa1917635
9. Zeiser R, Polverelli N, Ram R, et al; REACH3 Investigators. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228-238. doi:10.1056/NEJMoa2033122
10. FDA approves belumosudil for chronic graft-versus-host disease. Updated February 1, 2022. Accessed May 11, 2022. https://bit.ly/3NuPIEJ
11. Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study. Blood. 2021;138(22):2278-2289. Published correction appears in Blood. 2022;139(11):1772.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More