Roundtable Discussion: O’Brien and Participants Compare Therapies They Would Use in CLL
During a Targeted Oncology case-based roundtable event, Susan O'Brien, MD, discussed the case of a 71-year-old woman newly diagnosed with chronic lymphocytic leukemia.
Susan M. O’Brien, MD
Associate Director for Clinical Science
Chao Family Comprehensive Cancer Center
Medical Director
Sue and Ralph Stern Center for Clinical Trials and Research
Professor
Division of Hematology/Oncology, Medicine
School of Medicine
University of California, Irvine
Orange, CA


O’BRIEN: We’re just talking about risk in terms of disease risk, not necessarily risk based on comorbidities. Additionally, do you do molecular testing at your institution? Do you do IGHV mutation status? Do you get all these but send them out?
SHENOI: Usually when you see patients with CLL, they come through a consult, and you do the regular work-up, get a CLL FISH panel and an IGHV mutation [test], and most importantly, the flow [cytometry] to make the diagnosis. So that’s how I begin. If they have bulky lymph nodes and hepatosplenomegaly, I get an ultrasound as well; check the kidney function, [and] see how they are [and if they have] B symptoms or not.
Now, as you know, the majority of the patients are older, and they’re taken back by the diagnosis initially. I give them a little understanding of what the pathogenesis [is] and how it’s going to evolve. The majority of them do not need treatment up front, and that makes them more reassured. When I get to see them again about 7 to 10 days later, once the testing comes back—we do [our testing] in-house—I talk to them about their risk stratification. I still use the Rai staging system, even though it’s a little out of date, but I think that’s most reasonable for the community oncologist, not for research purposes.
Then I look at the risk factors. Are they young? Are they fit? Do they have comorbidities? But essentially, [I] look at the patient as a whole and then consider finite therapy vs infinite therapy depending on their functional status.
GOLDBERG: I think Dr Shenoi covered it. But most patients I see come in with lymphocytosis, they’re feeling well, and you can almost tell that you should wait and watch for a little while. We go through the molecular testing, which we send out, and the flow cytometry, etc, and it’s usually a pretty easy diagnosis to make.
HARRIS: I see a patient with early-stage Rai disease, 0 or 1, I often will not get all the prognostic testing up front. I’ll watch them carefully clinically, and if they start to have progressive lymphadenopathy, then I’ll get the immunoglobulin heavy chain and the FISH studies. I do the flow cytometry up front, but I might wait. I know that the IGHV tends to be immutable; it doesn’t change with progression of disease, but some of the other things do, like the cytogenetics. That’s why I would sometimes wait.
CHINTAPATLA: I pretty much follow your paradigm but mostly send out FISH at diagnosis to see the genetic status. [With] most patients, based on palpable lymphadenopathy, if I suspect any clinical lymphadenopathy increase, then I will do a CT scan on top of what everybody said. Not routine in all the cases.
SUN: Usually patients come in with lymphocytosis, and flow cytometry of peripheral blood usually confirms the diagnosis. Then I usually order a CLL FISH panel with cytogenetics and IGHV mutation up front. Usually I do not do a CT or ultrasound for lymphadenopathy unless they have a really bulky, palpable lymphadenopathy or splenomegaly. Usually I monitor. And for patients with B symptoms, usually I do imaging studies. But for the majority of patients, just flow cytometry, FISH, IGHV mutation, and cytogenetics up front.
KOSIEROWSKI: I oversee a number of states, with a number of local oncologists. But basically anyone who’s about to start therapy needs the complete work-up. Those patients who otherwise have relatively indolent disease can be watched for a while and save the million-dollar work-up until we decide to start therapy. But once they cross that line and need therapy, everyone gets the entire panel. We use a lot of send-out labs. A lot of them have a CLL profile that includes everything, so it makes it rather convenient.
GAO: I pretty much agree with what everyone just mentioned. Basically when we see a new patient, most of the time, they don’t need treatment. We will usually use a Rai stage and also send [out] flow cytometry for the diagnostic work. We usually don’t do the bone marrow until they need treatment.
I realize that sometimes you want to see if there’s any lymphadenopathy or some splenomegaly, and you want to do the CT scan. But a lot of time, the insurance won’t approve it, and that’s the reason I would say that I agree with Dr Sun, who mentioned if the patient does not have B symptoms, or other concerns, we usually would hold the further imaging studies.
With the flow cytometry, in the past, I found that they always reported CD38 and ZAP-70 as a prognostic purpose and information, but lately, I found that the flow cytometry no longer gives us those indexes. Sometimes we have to call to see if they can add it on. And in terms of the treatment, I would say that usually we would do the bone marrow before the treatment and send all the molecular studies, including the IGHV, TP53, or some FISH studies, to see if they have del(17p) or something like that.
ARORA: Yes, I think most people have covered what we do. I generally don’t do FISH studies until we are ready to treat them. I will do a marrow, FISH, and most of the studies at that time. But generally, I watch.
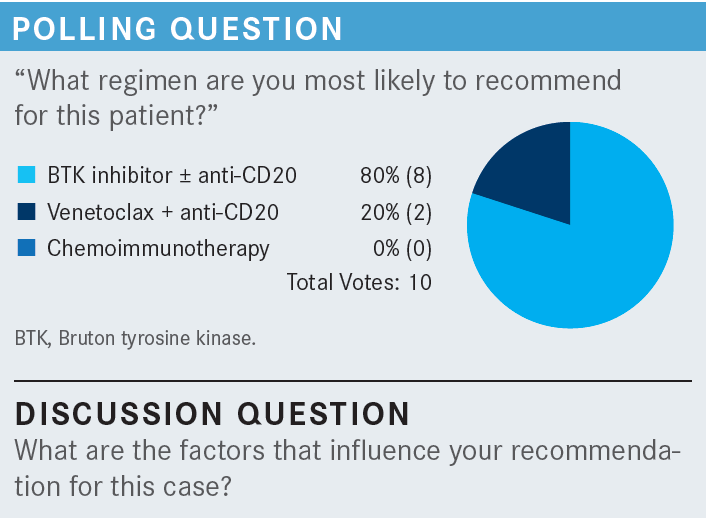
O’BRIEN: So tell me any or all factors that influence your recommendation for this case. Comorbidities, potentially, but her blood pressure was well controlled with medications, and she sometimes took a gastric acid blocker. Do the laboratory results matter—for example, the anemia that she had? What about her prognostic features? Age, performance status, frailty, etc? What would you use here, and what are the most important things in terms of making a decision?
HARRIS: Do we know if she had a Coombs test done? Whether this was an autoimmune hemolytic anemia?
O’BRIEN: That’s a really great point. We don’t have that in the case, but I think it’s quite relevant because I think what you’re alluding to, Dr Harris, is if that was your main indication for therapy and she was hemolyzing, perhaps you wouldn’t use any of those chemotherapies or any of those small molecule [inhibitors]. You’d treat the underlying hemolysis. But let’s say for the sake of discussion that she was not hemolyzing.
HARRIS: If this [case] is considering del(11q) high risk— and you were suggesting earlier that it may not be as high risk as del(17p)—but if, let’s say, she had a TP53 mutation as well, I would have considered giving her 3 months of a BTK [Bruton tyrosine kinase] inhibitor followed by a year of venetoclax [Venclexta], based on recent data that seems to suggest minimal residual disease after that [From the Data1]. I know that may be hard to get through insurance at this time, but it might be a reasonable alternative with bad prognostic factors. But I think I picked a BTK inhibitor alone [during the poll], to be followed by venetoclax if she relapses.

GOLDBERG: In a way, when you present this patient, I think the biggest decision is to treat or not to treat. And, notwithstanding all the prognostic signs, this woman’s quality of life seems to be affected. If we can give her a treatment that would increase her quality of life—she has anemia and bulky disease—I think all the other molecular tests reinforce your [decisions]. But I think this person should be treated.
SHENOI: I chose the venetoclax [and anti-CD20] combination, and the main reason for doing that is, as Dr Goldberg mentioned, she has a lot of life left in her….A finite therapy is what I would push for. [As for] the del(11q), usually those patients have bulky lymph nodes, and she only has lymph nodes in the axilla. But the LDH was not very elevated, only 250 U/L, so I don’t think she’s hemolyzing, as Dr Harris thought. But [we could try] prednisone. Just ordinary steroids can help. But in her case, she’s high risk by the mutation status as well, so a novel therapy such has venetoclax, a finite therapy, dose ramp-up 5 weeks, with obinutuzumab [Gazyva] or rituximab [Rituxan] up front, would do much better. That’s what I would do.
CHINTAPATLA: I chose the combination of venetoclax and anti-CD20. At presentation, she appeared frail. I think maybe a BTK inhibitor by single agent is not a bad option, but my reason to choose the venetoclax and anti-CD20 is the finite duration therapy, given the del(11q) mutation. If it is del(17p), I would have probably chosen BTK inhibition to begin with, but with the del(11q), I thought I probably would want to try a finite duration therapy.
THAPALIYA: Yes, I would probably choose some kind of a continuous treatment for her because with unmutated status, she is at a high risk of clonal evolution over time.
ARORA: My choice of therapy was pretty much based on her age, ease of therapy, what she can take, frailty, and need for therapy. I chose BTK inhibitors, and I would give it as a sole agent. I would have chosen acalabrutinib [Calquence] by itself, fewer adverse events. Finite vs infinite always plays on the mind. Cost plays on the mind. But that’s probably, for her, what I would have chosen.
YAN: I choose BTK too. I have to be honest. My knowledge about CLL is not very up-to-date, but I’m very comfortable with BTK inhibitors. When I look at the patient, I look at the age, comorbidities, and also the molecular risk. This is a patient who is 71 [years old], fragile, and has all the cytopenias, so she met criteria to be treated. So she is a very good candidate for the BTK inhibitor as a single agent.
GAO: I think we have agreement that this patient certainly needs treatment due to the symptoms, with weight loss, fatigue, and advanced-stage anemia, even [though] the thrombocytopenia is not that significant. Due to the risk, I will say that even her comorbidities seem [manageable]. She has hypertension. I know that BTK inhibitors sometimes can fluctuate the blood pressure, but as long as the patient’s heart is fine, my big concern would be the atrial fibrillation. I have at least 2 or 3 patients who experienced atrial fibrillation. I choose the BTK inhibitor based on the trial and also the tolerance, as well as the patient’s general condition.
SUN: I would choose the same: the BTK inhibitor. Generally, I choose acalabrutinib because of the reduced cardiac toxicity compared with ibrutinib [Imbruvica]. I have used zanubrutinib [Brukinsa] as well; it’s also well tolerated.

O’BRIEN: An interesting question here for our case discussion. So, for example, how many of you refer patients to a cardiologist to manage hypertension or atrial fibrillation? And do any of you have access to a cardio-oncologist, which is a field that has sprung up in the last 10 to 15 years?
GOLDBERG: We don’t have any cardio-oncologists. I would probably refer for atrial fibrillation, but not hypertension.
HARRIS: We have them at the University of Washington, locally. They’ve been mostly helpful in patients who have amyloidosis of the heart with multiple myeloma or other issues like that. But [we do not have them] locally in the county where we are.
O’BRIEN: Would you refer to a cardiologist for atrial fibrillation?
YAN: I manage all my patients myself if it’s straightforward, not hard. I’m still up-to-date with my medicine board. But if it’s complicated, I would. I would now, just because they have atrial fibrillation, so I automatically send them to cardiology. I think it depends.
SHENOI: I usually don’t do a consult, but I give them a call and just discuss it with them. But I do not get a consult just for that purpose.
O’BRIEN: How do you counsel your patients receiving a BTK inhibitor, in particular with respect to adherence, potential toxicities, drug-drug interactions? With the PPI [proton-pump inhibitor] and gastric [acid]–reducing agents, do you see as much use of the PPIs as I seem to? And do you routinely ask [patients] about that?
THAPALIYA: We have a patient education program before we start the patient on BTK inhibitors. So a physician assistant or a pharmacist would meet them in person or on a video chat and go over this before they started the treatment. We do office visits, so we make sure they’re compliant and monitor for any toxicities.
O’BRIEN: That’s handy. Do others have anything like that, or do they have to do that themselves?
GOLDBERG: We have either a nurse practitioner or physician assistant. They spend about an hour and a half for chemotherapy teaching any time we’re ready to start [a treatment]. It’s pretty [comprehensive].
CHINTAPATLA: We have a similar program with a nurse navigator, as well as the outpatient pharmacy.
SHENOI: I use an oncology nurse navigator, but I always discuss briefly, as we all should, before starting the drug so the patient knows what toxicities can occur.

O’BRIEN: Anybody want to comment on the PPI? Or is that something you haven’t paid much attention to? Because, with ibrutinib, it doesn’t matter.
ARORA: For the acalabrutinib, there is an interaction with PPIs, and I do see quite a few people on PPIs. I had a patient who I switched over to famotidine [Pepcid], but he still had significant issues with acid reflux. We had to bite the bullet and say, “We’ll watch your counts really closely, but we’ll put you on the PPI. But try and not take them together.” We’ll see.
O’BRIEN: I think somebody said earlier if that patient had del(17p), they’d be more inclined to use a BTK inhibitor.
So what if this patient had del(17p)? And is anybody using obinutuzumab with ibrutinib or acalabrutinib?
CHINTAPATLA: I commented that before, yes, if it is del(17p), I would probably use the BTK inhibitor.
O’BRIEN: Can you comment on why? I’m not disagreeing with you. I’m just curious as to what your thinking is.
CHINTAPATLA: I think del(17p)—we probably have longer-term data with the BTK inhibitors as opposed to the venetoclax regimen. Again, maybe they are the same as second-line therapies, but we just don’t have [those] data.
O’BRIEN: Whoever said they would use venetoclax in our case, can you say if you would use that for the del(17p) also?
SHENOI: That was me. I certainly would use a BTK for del(17p) because the data [are] much better for the del(17p). So yes, definitely. The question is, would I use a combination [regimen that included venetoclax]? The data are better, but the toxicity’s also a little more. And so using acalabrutinib with obinutuzumab could be a little tricky, depending on how the patient is.
O’BRIEN: Can some others comment on whether they have given [a monoclonal] antibody with a BTK inhibitor, number 1. And number 2, if they haven’t, are they now thinking about it?
HARRIS: Yes. With the 4-year follow-up data, I think it makes sense to consider that. Certainly, if the patient has additional toxicity, or more myelosuppression, or something of that nature, you may have to back off on the [monoclonal] antibody. But I think that it’s reasonable to approach most patients with that in mind.
GOLDBERG: I’m not sure that it’s worth doing. We have quite a few alternatives with the venetoclax. I’m not talking about the del(17p). But I think the difference with quality of life and CLL long-term survivals, maybe [you could use] short-term obinutuzumab—but I’m just not sure it’s worth the few extra points.
REFERENCE
1. Munir T, Moreno C, Owen C, et al. First prospective data on minimal residual disease (MRD) outcomes after fixed-duration ibrutinib plus venetoclax (Ibr+Ven) versus chlorambucil plus obinutuzumab (Clb+O) for first-line treatment of CLL in elderly or unfit patients: the Glow study. Blood. 2021;138(suppl 1):70. doi:10.1182/blood-2021-148666
