Roundtable Discussion: Ansell Discusses the Importance of New and Emerging Approaches in DLBCL
During a Targeted Oncology case-based roundtable event, Stephen Ansell, MD, PhD, discussed the options for third-line therapy for relapsed/refractory diffuse large B-cell lymphoma.
Stephen M. Ansell, MD, PhD
Consultant, Division of Hematology
Department of Internal Medicine
Chair of Faculty Development and Recruitment
Mayo Clinic
Rochester, MN


ANSELL: [Our case patient] had stage IV disease with high International Prognostic Index [IPI], and they initially did well with [6-cycles of] R-CHOP [rituximab (Rituxan), cyclophosphamide (Cytoxan), doxorubicin (Adriamycin), vincristine (Oncovin), prednisone] chemotherapy. Subsequently, they progressed and second-line treatment was with GemOx [gemcitabine (Gemzar), oxaliplatin (Eloxatin)] and rituximab where they got only a partial response [PR].
[The patient was also considered ineligible for transplant]. Now they are progressing, elderly, and not eligible for an autologous transplant.
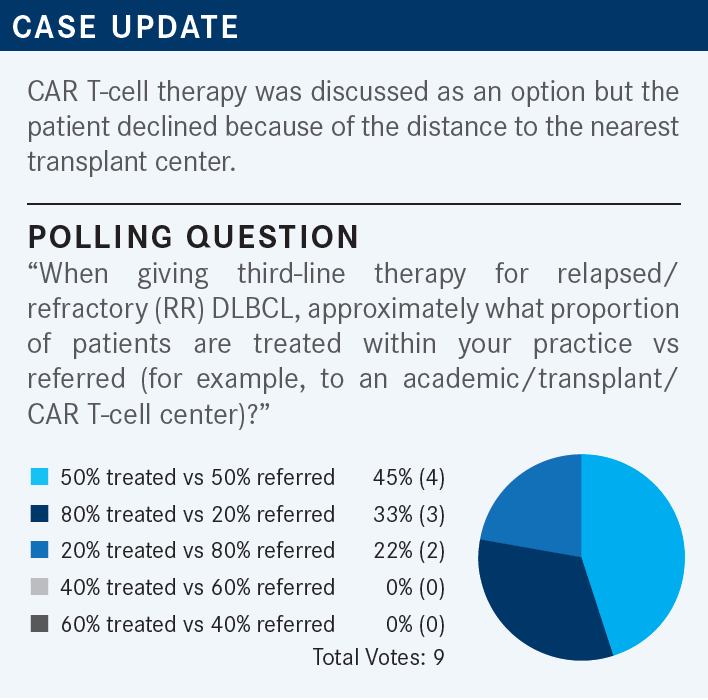
ARORA: I’m in a community private practice so, of course, the important issues are what kind of prior therapy the patient received. Comorbidities, performance status, and patient choice play the most important role. For me, the transplant center and chimeric antigen receptor [CAR] T-cell therapy center are quite close. It’s about 1 hour away and we have very good collegial relationships, so referral is quite easy.
ANSELL: I think those were good points. I think the availability of pretty much all options is interesting and good. As you pointed out, the patients’ preference is a key part and then comorbidities are an issue and prior therapies.
FLEJSIEROWICZ: I agree with what was said. It’s all the [factors listed]. Plus, possibly, the timing of relapse. Is it within 12 months or is it past 12 months? Could CAR T-cell therapy be a better idea for relapses within 12 months?
ANSELL: Let’s say the patient progressed in 6 months. How would you apply that to your decision-making?
FLEJSIEROWICZ: I think I would consider referring at this point, not only for the autologous transplant discussion but for CAR T-cell therapy consideration.
ANSELL: Are there other thoughts or comments as to your decision-making? Does the safety and tolerability of the regimen play into any of your decision-making at all? Or do you go, they’re all toxic or they’re not all that toxic, so you don’t mind?
BRIENDAR KUMAR: The toxicity matters a lot and, especially, in cardiac functions. A lot of these people can be elderly, which impacts their ability to tolerate treatment. [One should also] know how well they tolerated previous treatment. Treatment breaks also help decide how aggressive of a treatment to go forward with.
ANSELL: Do you pick 1 regimen or therapy depending on what you plan to use subsequently?
MORRISON: I think some of that might be related to if your center is a CAR T-cell therapy center, because if it is, it might be moved up a little bit earlier.
ANSELL: Certainly, in our practice that plays into things. If we’re thinking about CAR T-cell therapy earlier rather than later, we consider agents that have other targets on the tumor cell so that we don’t have some degree of potential overlap, although it’s not very clear that matters much. I guess 1 other thing that we would consider in our group is the goals of therapy. If it is to still try and cure the patient, would you consider a CAR T-cell therapy approach? Obviously, in this patient who is elderly and for whom transplant is not a consideration, but if you’re going for curative therapy, then, obviously, aggressive, defined treatment is what we’re shooting for.
ARORA: By the time the patient is getting second-line therapy, I already would have referred the patient to an academic center to get an opinion of how to treat the patient. Some of those patients may be eligible for transplant, some for CAR T-cell therapy, and some not. But at least by the second-line therapy, they’re already in the loop, so I have help from them.
ANSELL: Just as I’m looking at the poll, it’s interesting how it splits out into 3 groups. Some folks have 80% of patients that remain in the practice and only maybe 20% referred. Some had it at 50/50, and somewhere most of the patients are referred, just like Dr Arora was saying in his case. Again, thinking about a patient who had frontline treatment salvage therapy and is progressing now into the third line.
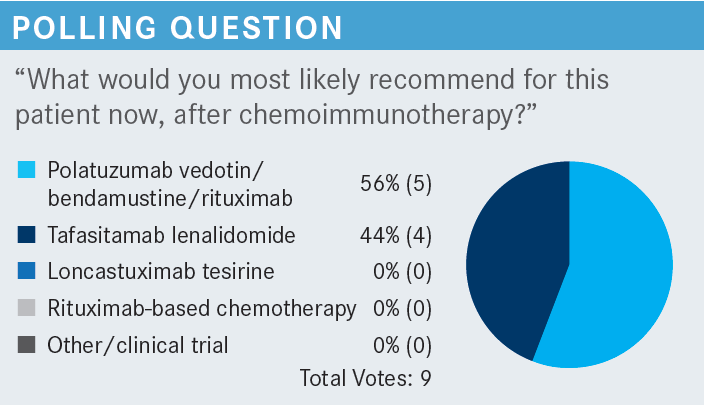
ANSELL: Do you have any comments or reflections on the use of tafasitamab [Monjuvi] and lenalidomide [Revlimid]? What are your thoughts on the merits and benefits of this combination?
ARORA: In my opinion, the tafasitamab plus lenalidomide regimen is much better tolerated and relatively easier to give. Clearly, being a non–chemotherapy-based regimen, it’s easy to tolerate for the patient also. Secondly, I have a question. The CR [complete response] rate is 40% in the second and third line. Why are we not moving [the tafasitamab plus lenalidomide] up? I do want to see that for some patients who are not candidates for R-CHOP. Perhaps they will be able to take this regimen in the first line and reap the great benefits. Because even in the third line, we have a CR of 40%.
ANSELL: I think you’re making a few good points. One of the things I mentioned earlier that is important in our practice, particularly in third-line therapy, is the durability of therapy. In other words, can they stay on the treatment, and is it tolerated well? Maybe with some dose modifications, they can remain on this treatment for an extended time. I think that’s very valuable.
Should we move it right into the frontline setting? I think the challenge is a significant percentage of patients across the board, somewhere around 45% or 50%, are cured with R-CHOP or R-CHOP–like regimens. The concern is always when you move [a regimen] into the frontline will you compromise cures? I think that’s the reason why you haven’t seen that happen to date.
ARORA: But for someone who is not eligible for R-CHOP and an elderly patient who doesn’t want to have chemotherapy, I think this is a perfect regimen and perhaps even lenalidomide can be dose-reduced. Tafasitamab can be continued indefinitely and lenalidomide stopped in 1 year. Again, what’s the logic? I don’t understand.
ANSELL: Again, trials don’t always follow complete logic. I think the sense was that tolerability would be more of a challenge. But I think you make a good point. For patients that are ineligible for standard therapy, this could be a reasonable option.
MORRISON: I agree. It’s relatively well tolerated. It’s not an issue with adjustments. It’s a great option for some patients who are not eligible for the other treatments. If you had somebody with a non–germinal center B-cell [GCB] subtype, would that influence which option you might use?
ANSELL: A good question. Maybe we could get comments from others. If you knew this patient had a non–GCB subtype, would that change your thinking about the regimen?
BRIENDRA KUMAR: I’ve not been deciding on treatment based on that.
PRIYA KUMAR: I don’t recall the inclusion criteria or the breakup of patients for the L-MIND [NCT02399085] and the polatuzumab [Polivy] [NCT02257567] studies. But I haven’t been deciding based on the cell of origin either.
JI: I would agree with both doctors. Mainly, the first-line treatment depends on whether the patient had a double-hit or triple-hit lymphoma. I may escalate the treatment to R-EPOCH [rituximab, etoposide (Toposar), prednisone, vincristine, cyclophosphamide, and doxorubicin] instead of R-CHOP. I’m not using cell of origin to decide what treatment I’m going to give. It depends on what the comorbidities are, the patient’s age, and whether the patient is a candidate for transplant or CAR T-cell therapy. That would help me decide on subsequent treatment.
ANSELL: I think you make a few good points and I think what you said resonates with me. Interestingly, if one looks at the use of lenalidomide with R-CHOP in the frontline, not all the studies show the dramatic difference that one would expect in the non–GCB subset. There was also substantial benefit in the GCB subset. I think it’s not as clear that it’s focused on one of the subtypes of lymphomas as much as what we might have considered. The other point that you made, Dr Ji, I think is important. When you have a multiply-relapsed patient with lymphoma, I think it doesn’t matter as much whether it is activated B-cell [ABC] or GCB. You have a bad disease. It keeps coming back and I think that may impact the therapy to a lesser degree. But Dr Morrison, I think that’s a good question and I think there’s not enough data, so we can debate that quite substantially.

ARORA: In my opinion, tafasitamab plus lenalidomide is the best-tolerated regimen and has shown a 40% CR rate in the second and third line. It is a non– chemotherapy-based option, so assuming there’s no restriction label or package insert, it would be my favorite. In my opinion, polatuzumab vedotin plus bendamustine is a relatively dirty regimen [as it has a significant amount of] adverse effects [AEs]. Of course, loncastuximab, is very impressive. It includes the trial of very high-risk patients with double-hit and triple-hit [lymphoma]. Some of the patients had transplants and CAR T-cell therapy and yet it showed some benefit and it’s a reasonably well-tolerated regimen. My first choice would be tafasitamab plus lenalidomide, my second choice would be loncastuximab, and my third choice would be polatuzumab vedotin plus bendamustine. I realize that polatuzumab vedotin is being investigated as a first-line drug in the R-CHOP combination [as well].
NARRA: Both tafasitamab and loncastuximab target CD19. Loncastuximab is an antibody-drug conjugate. What are your thoughts on tafasitamab vs loncastuximab in targeting CD19? Lenalidomide is CD20 targeted. That will be at play at some point. I’m not going to rule out polatuzumab plus bendamustine at any given point. I would like to bring in loncastuximab up front. What are your thoughts on that?
ANSELL: There’s always been some concern that if you target the same target on a tumor cell, you may compromise subsequent responses. Part of the worry was maybe that you’d get downregulation of the receptor and that would then make the target less viable. I don’t know that there’s any clear evidence that is entirely true. And in fact, there are some preliminary data that suggest that CD19 is very rapidly replaced on the cell surface. So I think the agents are very different.
When talking about loncastuximab and tafasitamab as far as their mechanism of action, one is delivering a cytotoxic agent and the other is relying on an immunological effect that is augmented by lenalidomide. I think they both have a place and they don’t negate each other’s effect. There’s a real possibility of moving these agents to frontline and I think we’re going to see trials with all these agents in the frontline in combination with more standard approaches. Or as Dr Arora mentioned, maybe in chemotherapy and particularly in anthracycline-ineligible patients, these agents may have a role.
CHHABRA: It looks like these are all phase 2 trials.1-3 It would be nice to have phase 3 randomized data, especially for tafasitamab and loncastuximab. I think that will give us more data and tell us how to sequence [the therapy]. In looking at the data, CAR T-cell therapy is the preference and if it can’t be done, then I think these 2 options are important. I would like to know the data on post–CAR T-cell therapy. It looks like there are some data with loncastuximab, but it’s a very small number of patients.
ANSELL: I think in patients who failed CAR T-cell therapy, the data are very thin. It’s just a very small number of patients. Even though polatuzumab does have some randomized data, it’s a randomized phase 2, not 3, trial. You’re quite correct. I think the data are still missing and that’s what the FDA, with accelerated approval, is requiring. Those studies are currently being developed and ongoing.
I will say this much, though. Most of us who treat these patients know that, unfortunately, none of these agents are the silver bullet to take care of everybody. Invariably, you end up rotating your way through these agents. There may be some merit to how you sequence them, but quite frankly, if we included even selinexor [Xpovio], we often find ourselves having to reach into each bucket and treat patients because, unfortunately, the disease recurs. The reason these are approved is purely because this is a tough-to-treat cohort of patients, and one ends up using many of these agents over time anyway.
BHAMIDIPATI: One comment I would like to make is [addressing] the peripheral neuropathy with polatuzumab vedotin. I have seen patients with peripheral neuropathy and patients who had GemOx prior or R-CHOP and usually, the tolerability of bendamustine can be tough. Dr Arora mentioned the polatuzumab vedotin regimen [and its AE profile], which I agree with. The cytopenia I have seen can be prominent by the time you get to the sixth round of polatuzumab vedotin plus bendamustine.
Again, tafasitamab may be a better option, in general, for durability. It’s tough in the initial 3 cycles where patients must go to the clinic quite often. It could be difficult for patients living far from the clinic, but once they get up to the fourth cycle or so, then it will be much easier. Personally, I do not have experience with using loncastuximab, but again, I have the same question whether you could sequence CD-19 agents followed by loncastuximab or something like that.
ANSELL: I think it does make a difference if you have a patient coming in with a degree of peripheral neuropathy from their previous vincristine. That may impact the potential AE profile of polatuzumab, for example.
REFERENCES
1. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
2. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
3. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:10.1016/ S1470-2045(21)00139-X
