Finn Reviews Options and Dosing for a Patient With Unresectable HCC
During a Targeted Oncology case-based roundtable event, Richard S. Finn, MD, discussed the case of a patient with hepatocellular cancer with a medical history of Crohn disease, cirrhosis, and variceal bleeding.
Richard S. Finn, MD
Professor of Medicine
Director, Translational Research Laboratory
Division of Hematology/Oncology
David Geffen School of Medicine at UCLA
UCLA Jonsson Comprehensive Cancer Center
Los Angeles, CA

Targeted OncologyTM: What do you think of this patient’s case? How does that affect your treatment choice?
FINN: This patient [has] some contraindications to atezolizumab [Tecentriq] plus bevacizumab [Avastin], which is the most active regimen we have in liver cancer based on its survival advantage, progression-free survival [PFS], response rate, safety, and tolerability. FOLFOX [folinic acid, fluorouracil, oxaliplatin] is not used in the United States. It is used sometimes in Asian populations but doesn’t have very strong data supporting it.1
Lenvatinib [Lenvima] is approved in the front line based on its noninferiority to sorafenib [Nexavar]. The REFLECT study [NCT01761266] compared lenvatinib with sorafenib for the primary end point of OS [overall survival]. It was noninferior but had superiority in objective response rate [ORR] and PFS, and there are some adverse event [AE] differences between sorafenib and lenvatinib. Nivolumab [Opdivo] is no longer approved in the United States as a single agent for liver cancer.
It was never approved as a frontline agent but was approved as second-line therapy and was withdrawn in late 2021 because the phase 3 study [NCT02576509] results of nivolumab vs sorafenib were negative. Sorafenib is still an approved frontline option [but is] probably being used less and less as we have more active agents.1 The 2021 NCCN [National Comprehensive Cancer Network] guidelines show atezolizumab plus bevacizumab as the preferred regimen, and then sorafenib and lenvatinib. Nivolumab was still listed, but it has a little note, which says [to use] “if ineligible for TKI [tyrosine kinase inhibitor] or other antiangiogenic agents.” So, if there’s nothing else to use, then maybe use nivolumab.
All the phase 3 studies in liver cancer have been concentrating on Child-Pugh A liver disease. That’s because patients with Child-Pugh B and C have less compensated liver disease and tend to die of their liver disease, not necessarily cancer, and it can be very hard to show that a drug improves survival for cancer if patients are dying of their liver disease. Nivolumab and other PD-1 or PD-L1 inhibitors are very well tolerated. There was a phase 1 expansion arm of nivolumab that looked very safe and had a similar ORR of [approximately] 15% as the Child-Pugh A population. I don’t know where FOLFOX would be used.1
What data support the use of sorafenib in patients with hepatocellular carcinoma (HCC)?
We have the SHARP study [NCT00105443]. This is the pivotal study that got sorafenib approved. It was published in the New England Journal of Medicine in 2008.2 It was a double-blind, placebo-controlled trial for patients who had advanced liver cancer and had never been treated with systemic treatment.
There were no effective systemic treatment back then and therefore patients were [randomly assigned] to sorafenib 400 mg twice a day or placebo. The primary outcome was OS and time to symptomatic progression, and there were other secondary end points.
The study [results showed] improved OS by [approximately] 3 months, from just under 8 months to 10.7 months. The hazard ratio [HR] was 0.69 [P < .001] or a 31% decrease in the risk of death, which is a respectable HR for cancer medicine. Interestingly, it improved survival with very few objective responses. It was pretty much a cytostatic effect, delaying progression, and the time to radiographic progression was [approximately] doubled with sorafenib. The common adverse events [AEs] with sorafenib are hand-foot and skin reaction, GI [gastrointestinal] toxicity, anorexia, and diarrhea. As a VEGF inhibitor it can cause some hypertension, but not too significant.2
How did lenvatinib affect this patient population?
The REFLECT study was the pivotal study that led to the approval of lenvatinib in 2018. It was the first drug for a decade to be approved in the frontline setting. Some drugs looked at [on trial were supposed to be] superior, and there were other drugs in noninferiority studies that were negative. This was a large study of [more than] 900 patients, and [as in] the SHARP study and others, the patients had Child-Pugh A and Barcelona stage B or C disease.3
Staging for liver cancer typically is not TNM [tumor, nodes, metastases], but [instead uses] the BCLC [Barcelona Clinic Liver Cancer staging] because it includes the risk of death from liver disease. If you just concentrate on tumor characteristics, you miss the other competing risks of death. Patients with Barcelona stage C have tumors that are extrahepatic or metastatic or those invading into the vasculature but their liver function is preserved; whereas those with Barcelona stage B have disease confined to the liver. This is a group that typically gets chemoembolization. They do not have vascular invasion. Both groups would be considered unresectable, right? Because they can’t be cured with a resection. All the systemic trials have some patients with intermediate disease.
The patients with stage B disease could get TACE [transarterial chemoembolization] and then progress after but not develop extrahepatic spread or vascular invasion. Some patients who present with intermediate disease just go straight to systemic treatment because the tumor burden is too large for local regional treatment. Importantly, this study excluded patients who had main portal vein invasion, a poor prognostic sign that means the tumor is growing out of the liver into the main portal vein. This was an open-label study of lenvatinib [8 mg or 12 mg based on weight] versus 400 mg of sorafenib [twice daily]. The primary end point was OS, and then other secondary end points [included] PFS.3
[The median OS was 13.6 months for lenvatinib and 12.3 months for sorafenib (HR, 0.92; 95% CI, 0.79-1.06)]. To be called noninferior, the prespecified upper limit would have been 1.08, and therefore this met its noninferiority end point [Figure3].
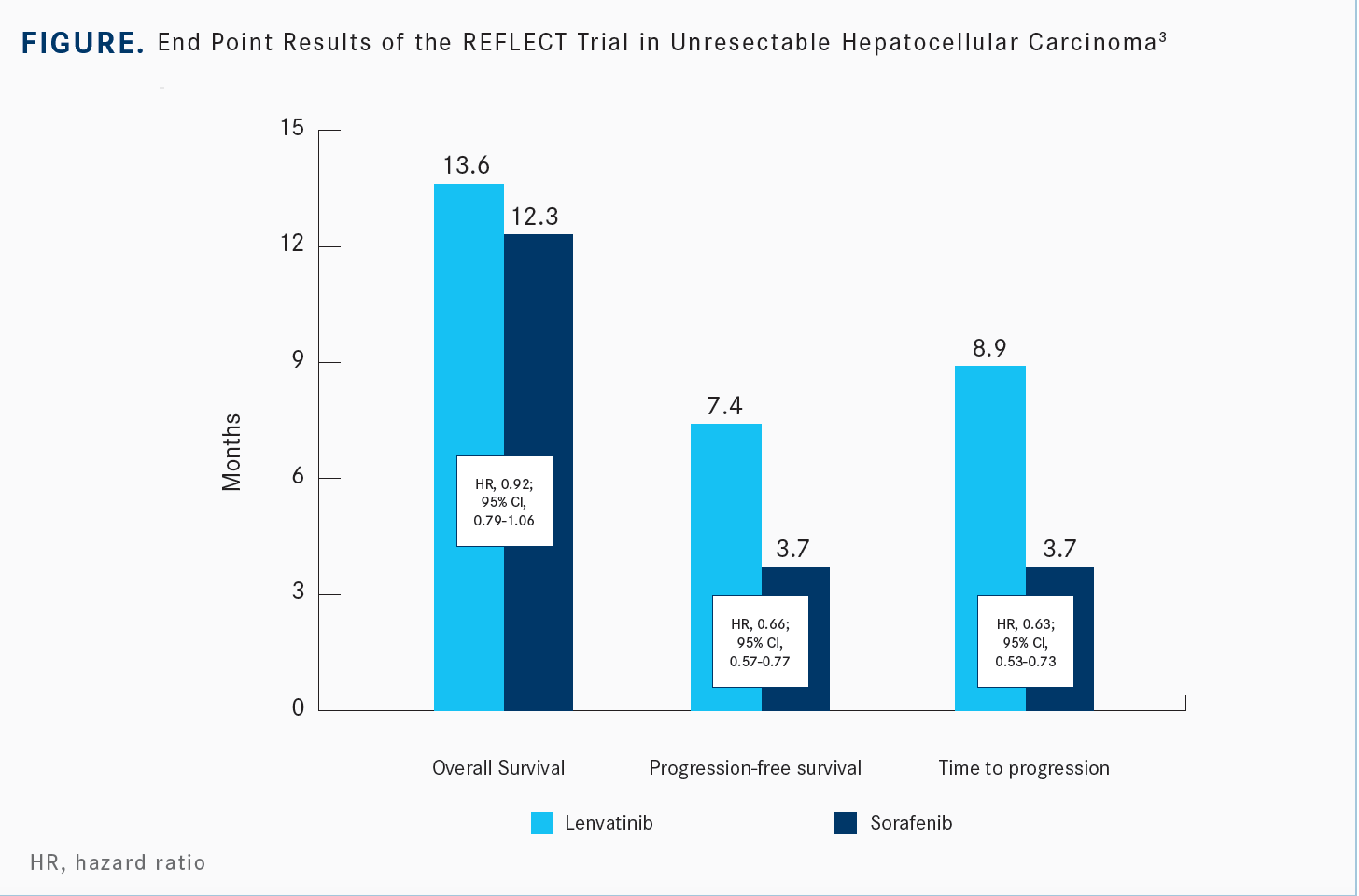
For secondary end points, the PFS was assessed by the modified RECIST approach, which looks at the enhancing characteristic of liver tumors. The PFS and TTP [time to progression] were essentially doubled with lenvatinib. Like sorafenib, lenvatinib is a very broad-spectrum VEGF receptor kinase inhibitor, but it’s much more potent against the VEGF receptor, but it also hits the FGFR receptors, so it differentiates itself a little bit. The HRs are 0.66 for PFS and 0.63 for TTP.
For the first time with a TKI, we have ORRs that are high. It’s 24% for lenvatinib with modified RECIST, but for sorafenib [it is 9%]. Even by conventional RECIST, we know the ORR was [approximately] 19% with lenvatinib.3
The forest plot for different subgroups tends to favor lenvatinib. The [subgroups] such as AFP and performance status are, in a way, prognostic of outcome. High AFP is worse than low AFP. Barcelona staging is also prognostic and patients with stage B disease do better than stage C, but both stages get a significant benefit with lenvatinib over sorafenib.3
I think this raises the question, especially now that we have more active drugs in liver cancer, about whether we need to start using systemic treatment earlier. That is to say that once a patient has received local regional treatment and at some point is no longer benefiting, we should not keep doing those treatments.
Before we had active drugs, interventional radiology filled a void, and we used it probably more than it was indicated, but now that we have so many active drugs I think it behooves us to keep an eye on patients. Should they not be getting benefit from TACE or Y-90 [radioembolization treatment], we should move on.
Many patients on the REFLECT study went on to receive additional treatments. We looked at this to see if the reason for the noninferiority could be that more patients on the sorafenib arm compared with the lenvatinib arm went on to other active drugs.
[Approximately] 61% of patients on the sorafenib arm did not get any more medication vs 68% [on the lenvatinib arm].3 We think part of the reason is because, [unlike sorafenib], lenvatinib was an investigational agent and patients could not go on to clinical studies.
How do the toxicity profiles of lenvatinib and sorafenib differ as used for unresectable HCC? What is your approach to AE prevention, monitoring, and management with each of these agents?
Both cause a similar incidence and grade of diarrhea and anorexia, but they are a little higher for lenvatinib. The bigger difference is when we look at hand-foot skin reaction, which occurs in higher frequency and grades with sorafenib. The converse is true for lenvatinib regarding hypertension, where there is more all grades and higher-grade hypertension.3 Our case patient had some anorexia and weight loss, which are probably the most common AEs that we need to react to.
There is no supportive medicine for that cruddy feeling. It’s very hard to work on appetite, and often a dose reduction or a short break is useful. I think lenvatinib mitigated some of their toxicities by dosing by weight. It’s the only TKI in liver cancer—and maybe other malignancies as well— that is dosed by weight, and I think that helps make it more tolerable.
The median duration of treatment was longer with lenvatinib [at 5.7 months compared with sorafenib at 3.7 months] because patients were getting more of a benefit. The PFS for lenvatinib was longer too. The discontinuation rate due to AEs was slightly higher with lenvatinib [at 13%] but was still comparable with sorafenib [at 9%]. The second-line therapies were more common with sorafenib.
In summary, [lenvatinib] was noninferior to sorafenib and showed improvements in PFS, TTP, and ORR. There were some health-related quality-of-life readouts that showed some delay in deterioration with lenvatinib vs sorafenib.
The indications for sorafenib and lenvatinib are very similar. Sorafenib is 400 mg twice a day. They’re 200-mg tablets, so you can go from 800 mg to 400 mg daily or 400 mg every other day, which is the typical recommended dosing strategy, though many of us will go from 400 mg twice daily to 200 mg twice daily in practice. The lenvatinib dose is 12 mg if the patient is over 60 kg and 8 mg if they’re lower. The dosing administration and contraindications are typical, and the boxed warnings are similar between both drugs.
What is your experience with AE management with TKIs and, specifically, lenvatinib?
The dosing recommendations include [monitoring for] hypothyroidism and proteinuria, which a patient cannot tell you about specifically. The starting doses [are 12 mg and 8 mg], and then it can be reduced by 4 mg. So if you start at 12 mg, you have a little more range, which can go down to 4 mg every other day. I think most patients can tolerate a dose somewhere in there.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Hepatobiliary cancers, version 1.2022. Accessed May 11, 2022. https://bit.ly/3Ao3MZW
2. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378- 390. doi:10.1056/NEJMoa0708857
3. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi:10.1016/ S0140-6736(18)30207-1

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More