Tafasitamab Plus Lenalidomide Shows Impressive Responses in Relapsed DLBCL
Zanetta Lamar, MD, discussed the efficacy of tafasitamab in combination with lenalidomide in the clinical trial setting.
Zanetta Lamar, MD

During a virtual Targeted Oncology Case-Based Roundtable event, Zanetta Lamar, MD, an oncology specialist at Florida Cancer Specialists and Research Institute, discussed the efficacy of tafasitamab in combination with lenalidomide in the clinical trial setting.

Targeted OncologyTM: What are the treatment options for this patient who experienced a relapse of cancer?
LAMAR: The NCCN [National Comprehensive Cancer Network] has different guidelines for transplant-eligible versus ineligible patients.1 In the guidelines for transplant- ineligible patients, for those who have received 2 or more therapies, options for therapy include gemcitabine [Gemzar] and oxaliplatin [GemOx], rituximab and GemOx, or polatuzumab vedotin [Polivy] plus bendamustine plus or minus rituximab. You can justify using just about any regimen for these patients. We know that tafasitamab[- cxix (Monjuvi)] plus lenalidomide [Revlimid] is approved for patients with relapsed/refractory DLBCL who are not eligible for transplant, but you can still pretty much use whatever regimen you want.
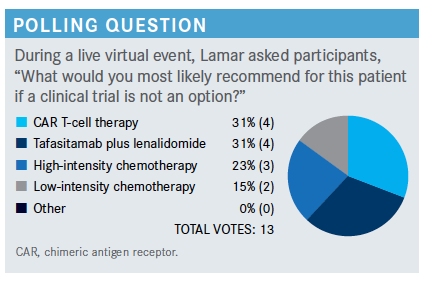

Please explain the rationale for combining tafasitamab and lenalidomide.
In July of last year, tafasitamab was granted approval by the FDA.2 Tafasitamab is a CD19-directed cytolytic antibody. It was approved in combination with lenalidomide for patients with relapsed or refractory DLBCL. That includes those with DLBCL arising from a low-grade lymphoma and those who are not eligible for transplant. The combination’s efficacy was evaluated in the L-MIND [NCT02399085] trial, an open-label, multicenter, single-arm study that included 81 patients. Tafasitamab was given intravenously and lenalidomide was given orally 21 out of 28 days.3
Both drugs were found to be active in lymphoma by themselves. Tafasitamab is an antibody-dependent cytotoxic drug. It induces cytotoxicity, and it induces phagocytosis. There was very good single-agent activity, but it was better in combination with lenalidomide.
What was the design of the phase 2 L-MIND study?
This was a single-arm trial that looked at patients with relapsed or refractory DLBCL. Patients had to have 1 to 3 prior regimens, so a patient who had more than 3 was excluded from this study.3 They could not have been eligible for high-dose therapy in transplant. Primary refractory patients were also excluded. However, when the trial first started, the definition of primary refractory changed. Initially, primary refractory was defined as relapse within 3 months of treatment. During the trial, that was amended to 6 months. Even though primary refractory patients were excluded, there were some who had refractory disease, but they were refractory within that 3- to 6-month window and were included in this study.
Tafasitamab was given during cycles 1 through 3 on a weekly basis. Lenalidomide was given for 21 out of 28 days. In cycles 4 through 12, tafasitamab was given twice monthly; beyond cycle 12, tafasitamab was given by itself twice a month. The primary end point was objective response rate [ORR].
When we look at the baseline characteristics, the median age [of patients] was 72 years old, and high and low IPI scores were pretty well balanced. Most patients in this study had stage III or IV disease, and most had an elevated LDH, with 2 or more lines of therapy. About 19 patients had primary refractory disease, and a little less than half [44%] were refractory to their last therapy. About 11% of patients had a transplant, and for the majority of patients, the cell of origin was unknown.
What were the efficacy outcomes of these data?
There were response rates at 17 months and at more than 2 years. There were not a lot of differences between 17 months and 2 years [34 patients with a complete response (CR) after 17 months and 33 at 2 years].3,4 The CR rate for the combination was a little over 40%, and the partial response rate was about 18%. About 15% had stable disease, and about that same number had progressive disease. The ORR was about 60%, and it was maintained over 2 years. The median duration of response at greater than 2 years was about 35%.
Median progression-free survival [PFS] [was reached at] 12.1 months, whereas median overall survival [OS] was not reached.4 PFS at [a follow-up] of 2 years was 16.2 months [95% CI, 6.3–not reached (NR)], and median OS at 2 years was 31.6 months [95% CI, 18.3-NR].
Were the adverse events (AEs) of this treatment mostly hematologic or nonhematologic? Which were the most serious?
Most were hematologic. Neutropenia, anemia, and thrombocytopenia were some of the more common AEs [experienced by at least 20% of patients on the study] and should almost be expected.3 [Grade 3 neutropenia] was experienced in about 27%. Nonhematologic AEs that occurred in more than 20% of patients included fatigue, diarrhea, cough, infection, and decreased appetite. [However, 21% of patients experienced grade 4 neutropenia.]
With serious AEs, again, a lot were hematologic, and about 12% of patients discontinued therapy due to treatment-related AEs. Some AEs of special interest were monitored, including dermatitis and basal cell cancers [Investigators] also evaluated whether some of these emergent AEs led to death. They did have 4 patients who died during this study.
Hematologic toxicity with neutropenia [was experienced the most on both treatment cycles]. During the first 12 cycles, when tafasitamab was combined with lenalidomide, there was a lot more toxicity.5 When tafasitamab was given as a monotherapy, a lot less in terms of AEs was seen, including [all grades of] neutropenia.
Did the study specify if the mortality rate was attributed to any treatment-related AEs?
The authors thought that deaths were not related to the medication. I think there was respiratory failure. There was 1 sudden death that they weren’t sure about, [and 1 patient had] a cardiac respiratory event. It’s not clear whether those were related to the medication, but they did happen during the course of this study.
References:
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 3.2021. Accessed April 14, 2021. https://bit.ly/3geoS5N
2. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed April 14, 2021. https://bit.ly/34Emq2z
3. Salles G, Duell J, Gonz.lez Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
4. Salles G, Duell J, Barca-Gonz.lez E, et al. Long-term outcomes from the phase 2 study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Abstract presented at: 2020 European Hematology Association Congress; May 14, 2020. Accessed April 15, 2021. https://bit.ly/3mVjq9a
5. Salles G, Duell J, Barca-Gonz.lez E, et al. Primary analysis results of the single-arm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Abstract presented at: 15th International Conference on Malignant Lymphoma; June 18-23, 2019; Lugano, Switzerland. Accessed April 15, 2021. https://bit.ly/3wZgJIt

Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen