Roundtable Discussion: Biran Leads a Conversation on Treatment Rationale in Multiple Myeloma
Noa Biran, MD, moderated a Targeted Oncology Case-Based Roundtable event during which time experts discussed treatment rationale in multiple myeloma.
Noa Biran, MD

Noa Biran, MD, Hematology/Oncology, Myeloma Division John Theurer Cancer Center Hackensack Meridian Health, moderated a Targeted Oncology Case-Based Roundtable event during which time experts discussed treatment rationale in multiple myeloma.

WU: I probably would not. I personally feel that even though bone marrow transplant would provide better outcomes, I’m concerned about the toxicity of the bone marrow transplant—and there are so many agents right now that we can use. Going by the literature, it makes sense. But I would have a discussion with the patient and provide options and see if [the patient] wants to go for a consultation with a bone marrow transplant physician.
BIRAN: That’s a great strategy. I agree there are so many options. Some people can get 5 years out of lenalidomide [Revlimid]/dexamethasone. If the person is 76 and they’re getting treatment, then certainly it has to be discussed as an option.
MURAKHOVSKAYA: I would at least collect the stem cells and have a discussion. I don’t think that transplant would be unreasonable in the space.
BIRAN: I think transplant offers an opportunity to have time off therapy. So even [though] it’s hard to get through, even if you’re going to follow it with low-dose lenalidomide or with nothing, that’s steroid-free time. It could be treatment-free time, and that could offer the patients a very good quality of life. The median without maintenance is 2. years. But with maintenance you can double that. Some patients get a decade of remission after transplant; 15% to 20% get a prolonged remission duration. I think that’s also a benefit—to be off therapy is important for quality of life.
According to the guidelines by IMWG [the International Myeloma Working Group], the recommendation is that the patients should be referred to a transplant center to determine eligibility, and chronological age and renal function should not be the sole criteria.1,2 There are a lot of misconceptions that [patients with renal dysfunction or on dialysis] cannot get transplanted. That isn’t true. Sometimes we need to reduce the melphalan dose or do inpatient stem cell mobilization. The collection can sometimes be more difficult than the transplant itself. But I think it’s a case-by-case situation, more on frailty of the patient and comorbidities rather than chronological age.
So who is eligible? Age is institution dependent; fitness, frailty, performance status, adequate organ function, concurrent amyloid doses involving the heart, and patient preference. Most centers will not transplant cardiac amyloid. The risk of mortality is high. They get ventricular arrythmias. But we usually have a risk-benefit discussion with these patients. Then patient preference—some patients don’t want to do it and that’s fine.
There are alternatives, as long as they know what the risks and the benefits are. Transplant is one of these things you cannot always defer. In a 76-year-old or a 77-year-old, you don’t harvest and hold. It’s hard to justify delaying transplant in these older patients. They tend not to get better with time. They tend to get worse with time. Most people who delay transplant don’t end up doing it—a large portion. So that’s something to think about.
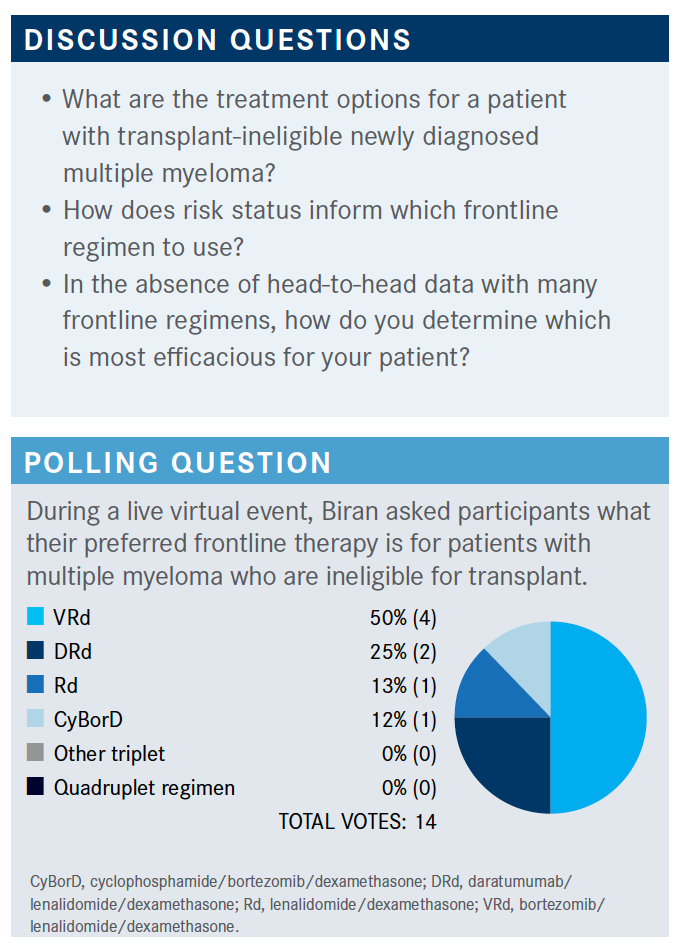
BIRAN: I think any of these [triplets] are appropriate. There’s a lot more variability in the transplant-ineligible population. I think it also depends on the patient. Even though we have a preferred therapy, everybody is going to change their mind depending on the comorbidities, the performance status, and the frailty of the patient. So all of these are reasonable.
Can someone say what they picked and why?
MO: I [chose VRd]. I think [overall survival [OS] is a concern across] multiple myeloma. So I chose VRd.
BIRAN:That’s a good option. Do you ever see any issues? Do you ever have to stop one of the drugs? Then what do you do once they’ve achieved remission? Do you stop the bortezomib [Velcade]? What’s your go-to in these patients?
MO: If a patient [has certain prognostic factors], I may prefer CyBorD [cyclophosphamide (Cytoxan)/bortezomib/dexamethasone], because the patient may [get afterwards] lenalidomide.
BIRAN: Great. Are you using twice-weekly bortezomib or once weekly?
MO: I usually at the beginning use it twice a week.
WU: VRd is what I prefer because [it gives an] extremely long PFS [progression-free survival]. I think VRd is a very good choice. Like Dr Mo said, I think that’s an excellent choice because that’s the only one that showed OS benefits.
BIRAN: The NCCN [National Comprehensive Cancer Network]-approved therapies for up front in nontransplant- eligible patients—all the ones you [all] mentioned are category 1: VRd [bortezomib/lenalidomide/dexamethasone], DRd [daratumumab (Darzalex)/lenalidomide/ dexamethasone], lenalidomide/dexamethasone alone, VCd [bortezomib/cyclophosphamide/dexamethasone]. Then you can also use other recommendations: KRd [carfilzomib (Kyprolis)/lenalidomide/dexamethasone], IRd [ixazomib (Ninlaro)/lenalidomide/dexamethasone], dara-BMP [daratumumab/bortezomib/ melphalan/prednisone], dara-VCd [daratumumab/cyclophosphamide/ bortezomib/dexamethasone]. Then, in circumstances that are special, you can use bortezomib/ dexamethasone, cyclophosphamide/lenalidomide/dexamethasone, and carfilzomib/cyclophosphamide/dexamethasone. These are all NCCN approved.3
Are there any patient disease- or treatment-related factors that you consider when you choose induction? I’ll give an example. I sometimes use a doublet up front in a very frail, older patient where I’m worried—sometimes I’ll give lenalidomide/dexamethasone alone as a very low dose because the bortezomib can sometimes be toxic in older patients. In high-risk patients, I will always give a triplet. Geographically, maybe we’ll avoid the bortezomib [if they live farther away]. I usually avoid carfilzomib in these older patients who are more frail; I like daratumumab in these patients. I think it’s very well tolerated, even in very old and frail patients; it’s convenient, and it’s very well tolerated and has a good depth of response.
KIM: So now that there’s oral melphalan, I don’t know whether that’s going to be moved up as a first-line [treatment].
BIRAN: Oral melphalan is the older one that’s around. There’s a new melphalan called melflufen. It’s intravenous. It’s approved for relapsed/refractory myeloma. It’s a peptide drug conjugate, so it’s a little fancier than melphalan. It injects the melphalan directly into the myeloma cells, so you’re theoretically getting a more potent targeted effect of the melphalan.
I haven’t used it yet, mostly because you need a central line to administer it and I don’t even know if it’s available. It just got approved. So we’ll see. I think there’s definitely going to be a role for it. There’s a signal in extramedullary disease for that drug. For patients who have soft tissue plasmacytomas, I think the response rate was around 15%, which isn’t extremely high but it’s something in that very refractory population.
BIRAN: What about cost? How does that factor in? Anybody have issues with getting these drugs? I have patients who say, “Look, even if it’s a co-pay, I’m not spending $3000 a month on lenalidomide.” Has anyone had issues with cost of drugs, oral drugs in particular?
STRAUSS: We have an in-house physician dispensing pharmacy. So if we have issues getting the co-pays down, we can get the co-pay assistance or often we can get foundations to help with our patients.
BIRAN: Great. So it’s not an issue for the most part?
STRAUSS: It’s more work for some of the pharmacy technicians. But we try not to let that affect [the patient].

BIRAN: [Studies of patients who have just received diagnoses] who are not transplant eligible [include the SWOGS0777 trial (NCT00644228), comparing VRd with Rd, and] this population was a little bit younger than we generally see.4
There’s [also] a single-arm VRd-lite trial, which looked at once-weekly VRd compared with twice-weekly [bortezomib].5 The response rate here was very high even with low-dose once-weekly bortezomib—86% response rate with 44% [complete response rate].
The ALCYONE trial [NCT02195479] was the first quadruplet trial, looking at the quadruplet of daratumumab-VMP [bortezomib/melphalan/prednisone] versus VMP alone, and again we’re seeing a very high response rate—91% with the quadruplet compared with 74% with the triplet.6
And then the one that’s the most applicable to our practice, at least from my perspective, is the MAIA study [NCT02252172], which looked at the combination of daratumumab/lenalidomide/dexamethasone versus lenalidomide/dexamethasone.7 Again, we’re seeing a higher response rate both with the doublet and the triplet; overall response rate of 93% [with daratumumab-Rd versus 82% with Rd] with a median PFS not reached compared with 34.4 months [for the triplet versus the doublet].
The ENDURANCE study [NCT01863550] was a study that compared VRd with KRd head-to-head in patients with newly diagnosed transplant-ineligible myeloma.8 This was an older population. Patients received lenalidomide maintenance therapy.
Patients who develop peripheral neuropathy and dropped out of study because of toxicity were not counted as a PFS event. So I think that grossly underestimated the PFS in the bortezomib arm [34.4 months vs 34.6 months with KRd].

BIRAN: PEGASUS is a retrospective study [of individual patient data from the Flatiron database] comparing real-world data [to that of the MAIA trial].9 [The study showed that] there’s a benefit in the addition of daratumumab even in real-world data.
I think [real-world data are] important. I think it’s good to see what people are doing in the real world because clinical trials don’t always translate into real patients. Because there’s a selection bias, these patients are well informed, they’re in good shape, and it doesn’t always translate. So, it’s always helpful to compare what we see in studies with what’s happening.
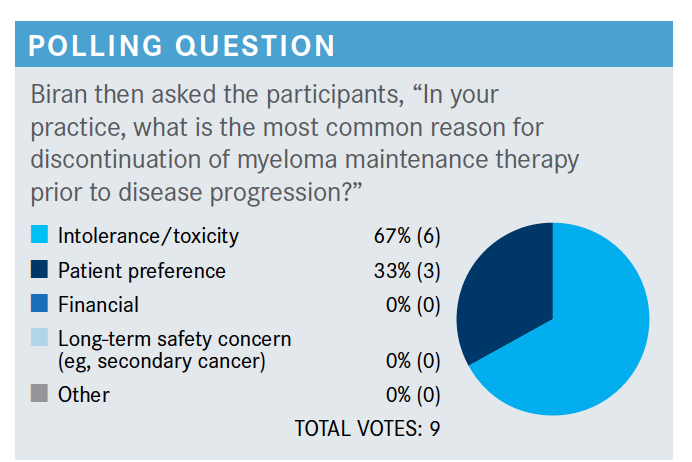
BIRAN: It looks like most people vote intolerance or toxicity or patient preference. I think there is a treatment fatigue that goes along with maintenance therapy that you [all] are referring to.
References:
1. Cavo M, Rajkumar SV, Palumbo A, et al; International Myeloma Working Group. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117(23):6063-6073. doi:10.1182/blood-2011-02-297325
2. Mikhael J, Ismaila N, Cheung MC, et al. Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. J Clin Oncol. 2019;37(14):1228- 1263. doi:10.1200/JCO.18.02096
3. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma; version 6.2021. Accessed April 18, 2021. https://bit.ly/3nje0Fh
4. Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. doi:10.1038/s41408-020-0311-8
5. Rodriguez C, Lantz J, Akbar F, Lantz L, Dressler E. Assessing efficacy and tolerability of a modified lenalidomide/bortezomib/dexamethasone (VRd- 28) regimen using weekly bortezomib in multiple myeloma. Presented at: 17th International Myeloma Workshop; September 12-15, 2019; Boston, MA. Abstract SP-036. Accessed April 18, 2021. https://bit.ly/32LTgg6
6. Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132-141. doi:10.1016/S0140-6736(19)32956-3
7. Facon T, Kumar S, Plesner T, et al; MAIA Trial Investigators. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104-2115. doi:10.1056/NEJMoa1817249
8. Kumar SK, Jacobus SJ, Cohen AD, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(10):1317-1330. doi:10.1016/S1470-2045(20)30452-6
9. Durie BGM, Kumar SK, Usmani SZ, et al. Daratumumab‐lenalidomide‐dexamethasone vs standard‐of‐care regimens: Efficacy in transplant‐ineligible untreated myeloma. Am J Hematol. 2020;95(12):1486-1494. doi:10.1002/ajh.25963

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More